Nature封面文章—突触研究重磅突破:科学家揭示了突触囊泡V-ATPase的工作原理
时间:2022-11-29 12:00:04 热度:37.1℃ 作者:网络
 中文摘要
中文摘要
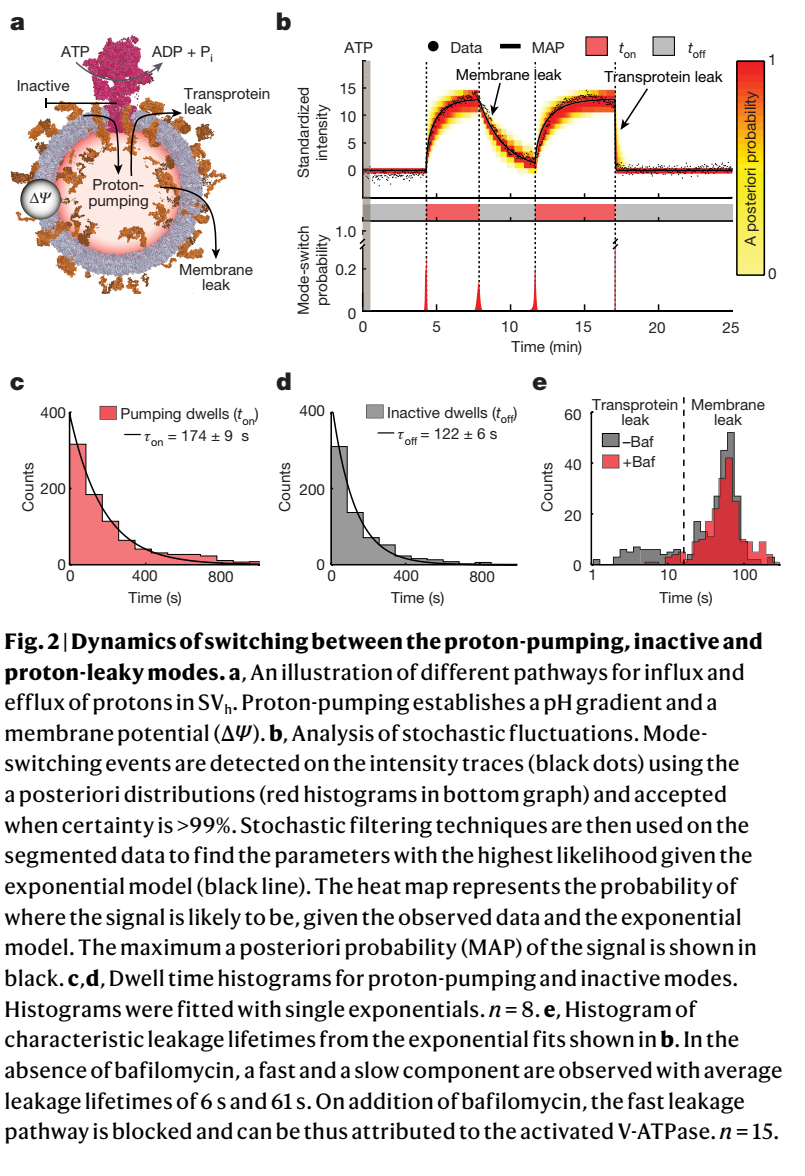
囊泡型腺苷三磷酸酶(V-ATPase)是一种与F型ATP合成酶结构相关的生电性旋转机械酶。它们水解ATP,为很多细胞过程建立电化学质子梯度。在神经元中,所有神经递质向突触囊泡中的装载都由每个突触囊泡的约一个V-ATPase分子提供能量。为了阐明这种单分子生物学过程,科学家研究了单个哺乳动物大脑V-ATP酶在单个突触囊泡中的生电质子泵。他们通过观察细菌同源物的旋转,并假设严格的ATP质子耦合,提出V-ATP酶不会在时间上连续的发挥“泵”的功能的观点。相反,他们认为V-ATP酶会随机地在三种超长寿命模式之间切换:质子泵、非活性和质子泄漏。值得注意的是,对泵活动的直接观察表明,ATP的生理相关浓度并不调节固有的泵工作的速率。ATP通过调控质子泵模式的切换概率间接影响V-ATP酶活性。相反,电化学质子梯度调节泵工作的速率以及泵的激活模式和非激活模式之间的切换。模式转换的直接后果是突触囊泡电化学梯度的全或无的随机波动,这可能会在质子驱动的神经递质装载过程中引入随机性,因此可能对神经传递产生重要影响。这项工作揭示并强调了超慢模式切换的机制和生物学重要性。
英文摘要
Vacuolar-type adenosine triphosphatases (V-ATPases)1-3 are electrogenic rotary mechanoenzymes structurally related to F-type ATP synthases4,5. They hydrolyse ATP to establish electrochemical proton gradients for a plethora of cellular processes1,3. In neurons, the loading of all neurotransmitters into synaptic vesicles is energized by about one V-ATPase molecule per synaptic vesicle6,7. To shed light on this bona fide single-molecule biological process, we investigated electrogenic proton-pumping by single mammalian-brain V-ATPases in single synaptic vesicles. Here we show that V-ATPases do not pump continuously in time, as suggested by observing the rotation of bacterial homologues8 and assuming strict ATP-proton coupling. Instead, they stochastically switch between three ultralong-lived modes: proton-pumping, inactive and proton-leaky. Notably, direct observation of pumping revealed that physiologically relevant concentrations of ATP do not regulate the intrinsic pumping rate. ATP regulates V-ATPase activity through the switching probability of the proton-pumping mode. By contrast, electrochemical proton gradients regulate the pumping rate and the switching of the pumping and inactive modes. A direct consequence of mode-switching is all-or-none stochastic fluctuations in the electrochemical gradient of synaptic vesicles that would be expected to introduce stochasticity in proton-driven secondary active loading of neurotransmitters and may thus have important implications for neurotransmission. This work reveals and emphasizes the mechanistic and biological importance of ultraslow mode-switching.