【论著】| 乳腺癌中心体调控蛋白SEC23B在肿瘤浸润转移中的作用及其机制研究
时间:2023-08-22 09:39:10 热度:37.1℃ 作者:网络
[摘要] 背景与目的:新的中心体调控蛋白Sec23同系物B(Sec23 homolog B,SEC23B)可以调节细胞中的自噬,从而提供有助于肿瘤生长和远处转移的能量和营养。然而,这种作用是否参与乳腺癌的浸润转移尚不清楚。本研究旨在探讨SEC23B在乳腺癌浸润转移中的作用及其分子机制。方法:使用Kaplan-Meier Plotter数据库和HCMDB数据库分析SEC23B表达与乳腺癌预后、转移之间的关联。将MCF-7细胞分为载体组(control)和SEC23B过表达质粒组(SEC23B),以及将MDA231-LM2细胞分为SEC23B敲低组(sh-SEC23B)和对照组(sh-NC)。采用蛋白质印迹法(Western blot)检测乳腺癌细胞中SEC23B、自噬相关蛋白[死骨片1(SQSTM1,p62)、微管相关蛋白1的轻链3(microtubule-associated-protein light-chain-3,LC3)]和细胞外调节蛋白激酶(extracellular-regulated protein kinases,ERK)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号转导通路蛋白的表达。通过transwell实验和伤口愈合实验评估细胞迁移,采用免疫荧光染色检测细胞中自噬颗粒。此外,构建体内腹膜内肿瘤模型研究SEC23B体内对乳腺癌细胞转移的影响。结果:SEC23B高表达的乳腺癌患者的总生存期明显短于SEC23B低表达的患者。SEC23B在转移性乳腺癌中的表达明显高于没有转移的乳腺癌原发性乳腺癌细胞(MCF-7、BT-549、MDA-MB-468和MDA-MB-453)中的SEC23B蛋白水平低于转移性乳腺癌细胞(MDA231-LM2和ZR-75-30)。与control组相比,SEC23B组明显加速了细胞迁移(P<0.001)。与sh-NC组相比, sh-SEC23B组明显降低了细胞迁移(P<0.001)。与control组相比,SEC23B组LC3A/B的表达水平和自噬颗粒的形成明显增加,而p62蛋白表达和mTOR、ERK的磷酸化水平降低。与sh-NC组相比,sh-SEC23B组LC3A/B表达水平和自噬颗粒的形成显著降低,和mTOR、ERK的磷酸化水平升高,特别是在饥饿条件下结果更显著。在体内实验中,与sh-NC组相比,sh-SEC23B组肿瘤重量显著降低(P<0.01),并且小鼠肿瘤组织中坏死组织增多和肺组织肿瘤转移减少。sh-SEC23B组小鼠肺转移肿瘤数和LC3免疫组织化学染色明显低于sh-NC组(P<0.01)。结论:SEC23B通过抑制ERK/mTOR信号转导通路来诱导乳腺癌自噬,并促进癌细胞转移。
[关键词] Sec23同系物B;乳腺癌;自噬;转移;ERK/mTOR信号转导通路
[Abstract]Background and purpose: The novel centrosomal regulatory protein Sec23 homolog B (SEC23B) regulates autophagy in cells, thereby providing energy and nutrients that contribute to tumor growth and distant metastasis. However, whether this role is involved in the mechanism of infiltrative metastasis in breast cancer is unclear. The aim of this study was to investigate the molecular mechanism by which SEC23B promotes breast cancer infiltration and metastasis. Methods: The association between SEC23B expression and breast cancer prognosis and metastasis was analyzed using Kaplan-Meier Plotter database and HCMDB database. MCF-7 cells were divided into vector group (control) and SEC23B overexpression plasmid group (SEC23B), and MDA231-LM2 cells were divided into SEC23B knockdown group (sh-SEC23B) and control group (sh-NC). Western blot was used to detect the expressions of SEC23B, autophagy-associated proteins [dead bone fragment 1 (SQSTM1, p62), microtubule-associated-protein light-chain-3 (LC3)] and extracellular-regulated protein kinases (ERK)/mammalian target of rapamycin (mTOR) pathway proteins in breast cancer cells. Cell migration was assessed by transwell assay and wound healing assay, and autophagic granules in cells were detected by immunofluorescence staining. In addition, an in vivo intraperitoneal tumor model was constructed to study the effect of SEC23B on breast cancer cell metastasis in vivo. Results: The overall survival of breast cancer patients with high SEC23B expression was significantly shorter than that of patients with low SEC23B expression. The expression of SEC23B in metastatic breast cancer was significantly higher than that in breast cancer without metastasis. SEC23B protein levels were lower in primary breast cancer cells (MCF-7, BT-549, MDA-MB-468 and MDA-MB-453) than in metastatic breast cancer cells (MDA231-LM2 and ZR-75-30). Compared with the control group, the SEC23B group significantly accelerated cell migration (P<0.001). Compared with the sh-NC group, the sh-SEC23B group significantly reduced cell migration (P<0.001). Compared with the control group, the expression level of LC3A/B and the formation of autophagy particles in the SEC23B group were significantly increased, while the expression of p62 protein and the phosphorylation levels of mTOR and ERK were decreased. Compared with the sh-NC group, the LC3A/B expression level and the formation of autophagy particles were significantly decreased in the sh-SEC23B group, and the phosphorylation levels of mTOR and ERK were increased, especially under starvation conditions. In in vivo experiments, tumor weight was significantly lower in the sh-SEC23B group compared with the sh-NC group (P<0.01), and there was an increase in necrotic tissue and a decrease in tumor metastasis in lung tissue in mice. sh-SEC23B group mice had a significantly lower number of lung metastatic tumors and LC3 immunohistochemical staining than the sh-NC group (P<0.01). Conclusions: SEC23B induces breast cancer autophagy and promotes cancer cell metastasis by inhibiting ERK/mTOR signaling pathway.
[Key word] Sec23 homolog B; Breast cancer; Autophagy; Metastasis; ERK/mTOR signaling pathway
乳腺癌是女性中最常见的肿瘤,并且转移是乳腺癌患者死亡的主要原因[1]。肿瘤转移是一个连续的、多步骤的病理学过程,需要细胞内外蛋白质共同参与[2]。在真核生物中,蛋白质分泌途径由一组细胞器和载货囊泡组成,该途径的第一步是由外壳蛋白Ⅱ(coat protein Ⅱ,COPⅡ)复合物将蛋白质从内质网转运到高尔基体[3]。Sec23同系物B(Sec23 homolog B,SEC23B)是COPⅡ复合物中的一种重要成分,它在COPⅡ组装和囊泡出芽中起作用,并参与调节蛋白质和脂质从内质网到细胞中高尔基体的运输[4]。先前的研究[5-7]表明,SEC23B异常激活可能会诱发癌症,并与甲状腺癌、肝细胞癌和前列腺癌的发展有关。最近研究[8]发现,SEC23B可以帮助调节细胞中的自噬。自噬是维持细胞稳态、调节细胞信号转导和促进细胞存活的关键细胞降解过程[9]。氧化应激在实体瘤中会诱导自噬,从而提供有助于肿瘤生长和远处转移的能量和营养[10]。因此,SEC23B是否通过介导自噬诱导肿瘤转移值得进一步研究。为了系统地探索SEC23B在自噬和肿瘤转移中的作用以及导致这些过程的潜在机制,我们进行了SEC23B的过表达或敲低实验来调节乳腺癌细胞中SEC23B表达,以探讨其对自噬、转移的影响。此外,我们使用了体内异种移植小鼠模型来证实SEC23B介导的自噬对乳腺癌细胞转移的影响。
1 材料和方法
1.1 细胞系、试剂和仪器
人乳腺癌细胞系MCF-7接种在DMEM培养基中培养,人乳腺癌细胞系MDA-MB-468和MDA-MB-453在Leibovitz's L-15中培养,人乳腺癌细胞系BT-549、ZR-75-30和MDA231-LM2在RPMI-1640(上述材料均购自上海源培生物科技股份有限公司)中培养。在37℃、CO2体积分数为5%的环境下,所有培养基均添加10%胎牛血清(fetal bovine serum,FBS)(购自美国Gibco公司)和50 IU青霉素/链霉素(购自上海源培生物科技股份有限公司)。
LipofectamineTM3000购自美国Invitrogen公司,针对SEC23B、肌动蛋白的一抗购自美国Proteintech公司,针对细胞外调节蛋白激酶(extracellular-regulated protein kinases,ERK)、p-ERK、哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)、p-mTOR、微管相关蛋白1的轻链3(microtubule-associated protein light-chain-3,LC3)A/B、死骨片1(SQSTM1,p62)的一抗购自美国CST公司,针对GAPDH的一抗购自美国Transgene公司,transwell板购自美国Corning公司,BCA检测试剂盒购自北京鼎国昌盛生物技术有限责任公司。
LAS 4000化学发光成像分析仪购自美国GE Healthcare公司,SP5共聚焦激光扫描显微镜购自德国Leica公司。
1.2 临床数据库分析
从Kaplan-Meier Plotter数据库中(https://kmplot.com/analysis/)获得2 477例SEC23B低表达和2 452例SEC23B高表达乳腺癌患者的生存数据分析比较。利用HCMDB数据库(https://hcmdb.i-sanger.com)箱线分析368例乳腺癌患者SEC23B表达与乳腺癌转移之间的关联。
1.3 细胞分组和转染
为了考察SEC23B对乳腺癌细胞迁移和自噬的影响,将MCF-7细胞转染SEC23B过表达质粒(SEC23B)来上调SEC23B表达,将MDA231-LM2细胞转染靶向SEC23B的化学修饰Stealth短发夹RNA(sh-SEC23B)以敲低SEC23B表达。使用LipofectamineTM3000将SEC23B、sh-SEC23B1或对照转染细胞48 h。
1.4 shRNA、质粒和转染
将细胞以3×104个细胞接种在6 cm培养皿中,并在不含抗生素的培养基中培养24 h。靶向SEC23B的化学修饰Stealth短发夹RNA(sh-SEC23B)和对照(sh-NC),以及Flag-SEC23B质粒编码N末端带有Flag标记的SEC23B蛋白(SEC23B)和对照(control)购自广州Ribobio公司。根据产品说明书,使用LipofectamineTM3000将siRNA和质粒转染到细胞中。用终浓度为20 nmol/L的siRNA转染细胞。在转染48 h后,收集细胞通过蛋白质印迹法(Western blot)分析转染效率。
1.5 Western blot检测
细胞用PBS洗涤两次,并置于RIPA裂解缓冲液[150 mmol/L NaCl、0.1% SDS、50 mmol/L Tris-HCl (pH为7.5)、1% NP-40、1%脱氧胆酸钠]中,在冰上裂解20 min。使用BCA检测试剂盒测量每个样品的蛋白质浓度。随后,将聚丙烯酰胺凝胶电泳(polyacrylamide gel electrophoresis,PAGE)分离的蛋白质转移到硝酸纤维素膜上,然后分别与各种抗体一起温育。用于Western blot检测的抗体用含有0.01%叠氮化钠和5%牛血清白蛋白的缓冲液以不同比例稀释。使用LAS 4000化学发光成像分析仪令Western blot检测可视化。使用的抗体包括针对SEC23B(1∶500)、p-ERK(1∶500)、ERK(1∶500)、p-mTOR(1∶800)、mTOR(1∶800)、LC3A/B(1∶500)、p62(1∶500)、肌动蛋白(1∶5 000作为阴性对照)和GAPDH(1∶6 000作为阴性对照)的一抗。所有Western blot检测进行3次进行。用Image J软件对条带强度进行量化。
1.6 Transwell检测
将2×104细胞用无血清DMEM洗涤,悬浮在0.2 mL无血清DMEM中,并添加到transwell小室的上层(8 μm孔径,24 孔板)。随后将0.5 mL含有20%FBS的DMEM添加到下室中。将腔室在37℃下温育24 h后,将滤膜用甲醇固定10 min,并用0.1%结晶紫染色15 min。用棉签轻轻去除上室的细胞。在显微镜下以100×放大倍数捕获下室迁移的细胞,并在5个随机视野中计数。每个实验至少重复3次。迁移率标准化为对照组。
1.7 划痕试验
在刮擦前一天将细胞接种在6孔板中,以获得大约100%的细胞密度。用200 μL移液器吸嘴进行直划痕,然后用PBS洗涤细胞2次并用RPMI-1640培养基培养。每12 h记录1次划痕的长度。
1.8 免疫荧光染色
载玻片上的细胞在室温下用4%多聚甲醛固定15 min。随后,用PBS冲洗两次后,用0.4% Triton-X100透化细胞30 min,并在室温下用5%BSA封闭1 h,然后将细胞与抗GFP-LC3的一抗按1∶100比例在4℃下温育过夜,与二抗在室温下温育1 h,然后用DAPI复染。使用SP5共聚焦激光扫描显微镜获取图像,并使用Image J进行分析。
1.9 异种移植瘤模型
无特定病原体(SPF)级雌性BALB/c裸小鼠(15~20 g,6~8周龄)购自湖南斯莱克景达实验动物有限公司(许可号:SCXK 2019-0004)。所有小鼠都安置在平均恒温(23±2℃)的房间中,光照周期为12 h,相对湿度为50%~60%,并且可以自由获取标准颗粒饲料和水。实验前将小鼠在这些环境中适应性喂养至少1周。将所有小鼠随机分为2组(n=6):sh-NC组和sh-SEC23B组。将具有稳定SEC23B敲低(sh-SEC23B)或阴性对照(sh-NC)的MDA231-LM2细胞(1×106个细胞)腹膜内注射到小鼠中。3周后,将小鼠在麻醉下处死。切除肿瘤和肺组织,分别在4%多聚甲醛和布因氏液中固定,用于进一步分析。
1.10 H-E染色和免疫组织化学染色
将肿瘤和肺样本包埋在石蜡中以制备4 μm厚的切片,用苏木精和伊红(hematoxylin and eosin,H-E)染色,并在光学显微镜下观察。对于免疫组织化学染色,将肿瘤切片用二甲苯脱蜡并用梯度乙醇水化,然后使用0.01 mol/L柠檬酸盐缓冲液进行抗原和抗体修复。切片用3% H2O2处理,然后在室温下用10%山羊血清封闭30 min。LC3(1∶200)一抗在4℃温育过夜,二抗在室温温育30 min。使用DAB进行显色后,将载玻片用苏木精复染2 min,然后在光学显微镜下观察。
1.11 统计学处理
所有数据均使用SPSS 21.0进行处理。连续性变量数据描述为x±s,两组间比较使用Student’s t检验(双尾)检验分析实验结果,多组间比较采用单因素方差分析方法分析实验结果。P <0.05为差异有统计学意义。
2 结 果
2.1 SEC23B在转移性乳腺癌中上调
为了评估SEC23B在乳腺癌中的作用,通过Kaplan-Meier分析了SEC23B表达水平与乳腺癌患者存活率之间的相关性。SEC23B高表达的乳腺癌患者的总生存期明显短于SEC23B低表达的患者(图1A)。此外,在临床数据库HCMDB中分析了有或无转移的乳腺癌中的SEC23B表达。SEC23B在转移性乳腺癌中的表达明显高于没有转移的乳腺癌,表明SEC23B高表达是乳腺癌预后不良的标志物。然后,在从原发性或转移性乳腺癌中分离的可用乳腺癌细胞系中分析SEC23B蛋白水平(图1B)。乳腺癌细胞系(MCF-7、BT-549、MDA-MB-468和MDA-MB-453)中的SEC23B蛋白水平低于高转移潜能乳腺癌细胞系(MDA231-LM2和ZR-75-30,图1C)。

图1 SEC23B在转移性肝癌中表达上调
Fig. 1 SEC23B is upregulated in metastatic breast cancer
A: Kaplan-Meier survival analysis of the correlation between SEC23B expression levels and overall survival of breast cancer patients was performed in an online database (n=4 929); B: Association between SEC23B expression and breast cancer metastasis was analyzed using the HCMDB database box line (n=368); C: SEC23B expression in breast cancer cells was detected by Western blot.
2.2 SEC23B在体外促进乳腺癌的迁移
为了进一步研究SEC23B在乳腺癌中的功能,在MCF-7细胞中分析了过表达SEC23B对细胞迁移的影响(图2A)。Transwell和划痕实验结果显示,与control组相比,SEC23B组明显加速了细胞迁移和伤口愈合(P<0.001,图2B、C)。此外,在MDA231-LM2细胞中敲低了SEC23B(图2D),与sh-NC组相比,sh-SEC23B组明显降低了细胞迁移和伤口愈合(P<0.001,图2E、F)。
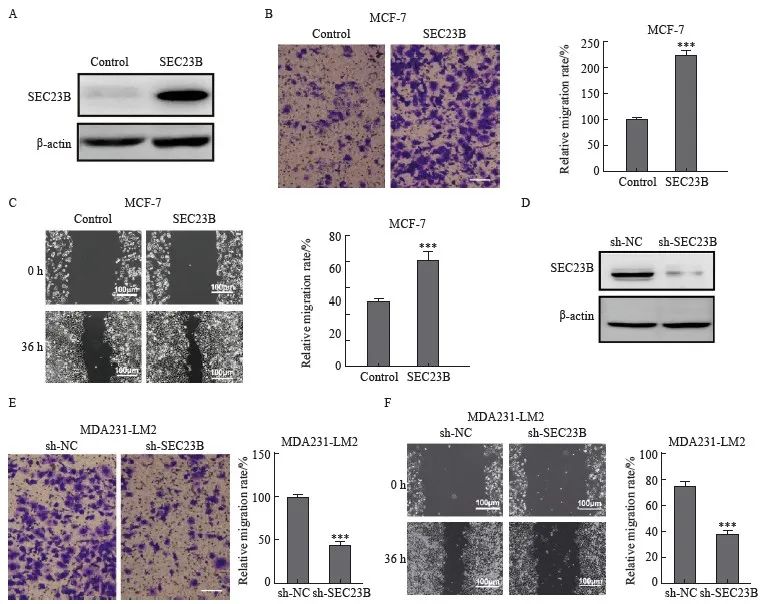
图2 SEC23B在体外促进乳腺癌细胞的迁移
Fig. 2 SEC23B promotes breast cancer migration in vitro
A: Transfection efficiency of SEC23B overexpression plasmid in MCF-7 cells was examined by Western blot; B-C: Effect of SEC23B overexpression on MCF-7 cell migration was assessed by transwell assay and wound healing assay; D: The expression of sh-SEC23B lentivirus in MDA231-LM2 cells by Western blot; E-F: Effect of SEC23B knockdown on migration of MDA231-LM2 cells was assessed by transwell assay and wound healing assay. ***: P<0.001, compared to control or sh-NC groups.
2.3 SEC23B诱导乳腺癌细胞自噬
为了探索SEC23B是否参与自噬,本研究在抑制或过表达SEC23B后检测了乳腺癌细胞系MCF-7和MDA231-LM2中自噬标志物LC3的变化。与control组相比,SEC23B组LC3A/B的表达水平增加,而p62蛋白表达降低(图3A)。免疫荧光实验结果显示,SEC23B组自噬颗粒的形成较control组显著增加(P<0.01)(图3B)。相反,与sh-NC组相比,sh-SEC23B组LC3A/B表达水平和自噬颗粒的形成降低,在饥饿条件下更为显著(P <0.01,图3C、D)。结果表明,SEC23B可促进乳腺癌细胞中的自噬。本研究进一步分析了抑制自噬对乳腺癌细胞迁移的影响,采用自噬抑制剂CQ处理过表达SEC23B的MCF-7细胞,结果显示,与PBS组相比,CQ组细胞迁移显著降低(P <0.001,图3E)。
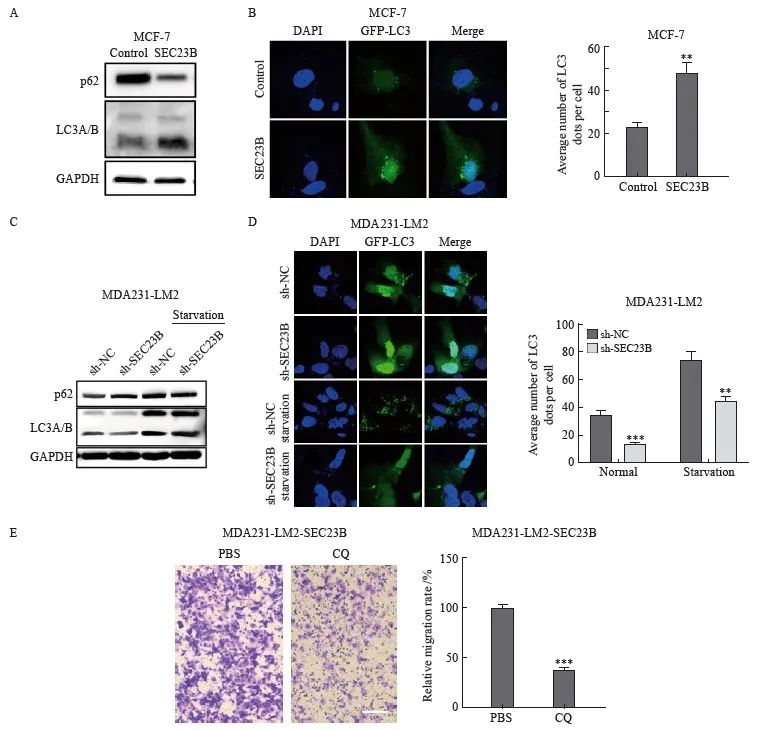
图3 SEC23B诱导乳腺癌细胞自噬
Fig. 3 SEC23B induces autophagy in breast cancer cells
A: Effect of SEC23B overexpression plasmid on p62, LCA/B expression in MCF-7 cells detected by Western blot; B: Representative images and quantitative analysis of the effect of SEC23B overexpression on autophagic granules in MCF-7 cells detected by immunofluorescence staining; C: Effect of SEC23B knockdown on p62, LCA/B expression in MDA231-LM2 cells by Western blot; D: Representative images and quantitative analysis of the effect of SEC23B knockdown on autophagic granules in MDA231-LM2 detected by immunofluorescence staining; E: Effect of CQ on migration of SEC23B overexpressing MCF-7 cells was assessed by transwell assay. **:P<0.01, compared with control or sh-NC groups; ***: P<0.001, compared with control or sh-NC groups.
2.4 ERK/mTOR信号转导通路参与SEC23B在乳腺癌细胞中诱导的自噬
ERK/mTOR信号转导通路是影响自噬的关键因素,因此我们推测该信号转导通路可能参与了SEC23B在乳腺癌细胞中诱导的自噬。SEC23B上调后,MCF-7细胞中mTOR和ERK的磷酸化水平降低(图4A)。相反,SEC23B敲低后,MDA231-LM2细胞中mTOR和ERK的磷酸化水平升高,特别是在饥饿条件下(图4B)。以上结果表明ERK/mTOR信号转导通路可能参与SEC23B诱导的乳腺癌细胞自噬。为了进一步证明SEC23B通过ERK/mTOR信号转导通路影响自噬,我们用ERK激动剂表皮生长因子(epidermal growth factor,EGF)处理SEC23B过表达的MCF-7细胞,结果显示, EGF抑制了SEC23B诱导的LC3A/B和p62的蛋白表达水平变化(图4C)。免疫荧光实验结果显示,与SEC23B组相比,SEC23B+EGF组的自噬颗粒形成显著降低(P <0.05,图4D)。这些结果表明,SEC23B通过影响ERK/mTOR信号转导通路来诱导乳腺癌细胞自噬。
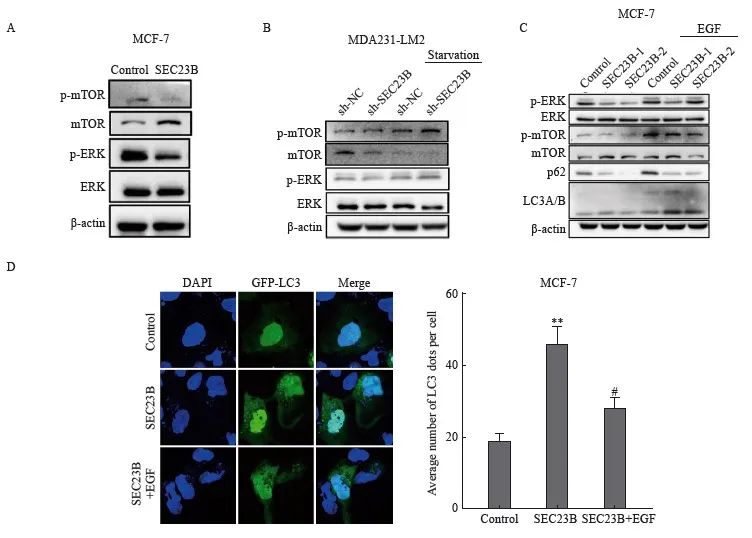
图4 ERK/mTOR信号转导通路参与SEC23B在乳腺癌细胞中诱导的自噬
Fig. 4 ERK/mTOR pathway is involved in SEC23B-induced autophagy in breast cancer cells
A: Effect of SEC23B overexpression plasmid on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot; B: Effect of SEC23B knockdown on ERK/mTOR pathway expression in MDA231-LM2 cells detected by Western blot; C: Effect of EGF on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot, effects of EGF on ERK/mTOR pathway and p62, LCA/B expression in SEC23B overexpressing MCF-7 cells detected by Western blot; D: Representative images and quantitative analysis of the effects of EGF on autophagic granules in SEC23B overexpressing MCF-7 cells detected by immunofluorescence staining. **:P<0.01, compared with the control group; #: P<0.05, compared with the SEC23B group.
2.5 SEC23B敲低在体内抑制乳腺癌转移和自噬
为了进一步验证SEC23B对移植瘤的影响,小鼠皮下注射了具有稳定SEC23B敲低(sh-SEC23B)或阴性对照(sh-NC)的MDA231-LM2细胞。与sh-NC组相比,sh-SEC23B组肿瘤体积更小,并且肿瘤重量显著降低(P<0.01,图5A)。H-E染色结果显示,与sh-NC组相比,sh-SEC23B组小鼠肿瘤组织中坏死组织增多(图5B),并且肺组织肿瘤转移减少(图5C)。取肺组织进行Bouin固定,sh-SEC23B组小鼠肺转移肿瘤数明显低于sh-NC组(P<0.01,图5D)。此外,sh-SEC23B组小鼠肿瘤组织中LC3免疫组织化学染色明显低于sh-NC组(P <0.001,图5E)。结果表明,SEC23B可以作为致癌基因在体内促进乳腺癌细胞生长、转移和自噬。
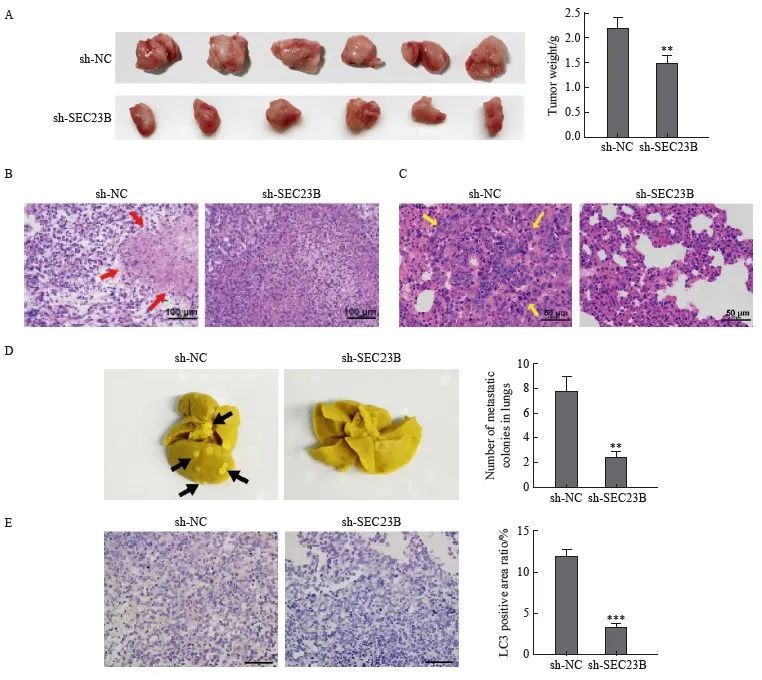
图5 SEC23B敲低在体内抑制乳腺癌转移和自噬
Fig. 5 SEC23B knockdown inhibits breast cancer metastasis and autophagy in vivo
A: Tumors were excised 21 days after implantation and final tumor weight was recorded. B: Histological examination by H-E staining was performed to determine tumor morphology and structural changes. Tumor necrosis was indicated by a red arrow. C: Lung metastases were examined by H-E staining. Tumor metastases in lung tissue were indicated by yellow arrows. D: Lung tissue was excised and fixed in Bouin solution 21 days after implantation, and the number of metastatic nodules was recorded for each mouse. Metastatic nodules were indicated by black arrows. E: LC3 expression in tumor tissues was examined by H-E staining. **:P<0.01, compared with sh-NC group; ***: P<0.001, compared with sh-NC group.
3 讨 论
本研究探讨了SEC23B在促进乳腺癌转移中的潜在机制以及SEC23B在自噬中的潜在作用。数据库分析结果显示,SEC23B高表达的患者生存时间较短,并且与肿瘤转移有关。在体外实验中,SEC23B增强了乳腺癌细胞的迁移能力并诱导了自噬。我们的研究揭示了SEC23B促进乳腺癌进展和抑制自噬的新机制,这可能为乳腺癌治疗提供新的见解。
SEC23B是一种GTP酶激活蛋白,可刺激SAR1-GTP水解以促进体内囊泡运输[4]。研究[11]表明,SEC23B基因突变导致先天性红细胞生成异常性贫血Ⅱ型。迄今为止,关于SEC23B异常表达与人类癌症发展的报道较少。研究[12]发现,SEC23B上调能够促进正常甲状腺细胞的增殖、集落形成、存活和侵袭,这与甲状腺癌诱发的癌症易感性有关。另一项研究[13]表明,SEC23B通过核糖体生物合成途径导致癌症易感性。此外,一项串联质谱分析发现,SEC23B表达在肝肿瘤大鼠内质网中上调,提示SEC23B可用作肝细胞癌的潜在新型肿瘤标志物[7]。有研究[6]证实SEC23B是miR-130a的靶标,后者在前列腺癌中下调,而敲低SEC23B表达模拟了miR-130a在前列腺癌细胞中过表达的影响。与这些研究报道的结果一致,本研究发现SEC23B过表达促进了乳腺癌细胞的转移,而SEC23B沉默抑制了体内肿瘤的生长和转移。这些结果证实SEC23B在乳腺癌的进展中起关键作用。
自噬在肿瘤发生或抑制中发挥双重作用[14]。在肿瘤发生的早期,自噬作为一种肿瘤抑制因子,可以降解肿瘤细胞中的增殖相关蛋白和结构底物,并激活受损细胞中的程序性细胞死亡[15]。然而,随着肿瘤的生长,氧化应激在实体瘤中诱导自噬,从而提供有助于肿瘤生长和远处转移的能量和营养[10]。相比之下, LC3B的高表达与肿瘤侵袭和转移有关,并提示预后不良[16]。在本研究中,我们发现SEC23B促进了乳腺癌细胞中的自噬,并且用自噬抑制剂CQ处理过表达SEC23B的MCF-7细胞后,细胞转移能力降低。研究[17]发现,营养缺乏会导致SEC23B重新定位于内质网高尔基体中间区,促进自噬通量。此外,还有研究[18]发现SEC23B参与细胞内COPⅡ囊泡的形成,这是自噬过程中自噬体生物发生的关键步骤。这些结果提示,SEC23B可能通过诱导乳腺癌细胞自噬促进癌细胞转移。
自噬可以通过不同的信号转导通路调节,尤其是mTOR信号转导通路,它可以由MAPK/ERK信号转导通路调节[19]。本研究发现SEC23B抑制了乳腺癌细胞的ERK/mTOR信号转导通路。SEC23B通过抑制PPP1CA介导的ERK/MAPK信号转导通路促进结直肠癌的生长和转移[20]。本
研究还发现ERK激动剂EGF处理过表达SEC23B的MCF-7细胞后,细胞自噬水平降低。这些结果表明SEC23B至少部分地通过抑制ERK/mTOR信号转导通路激活乳腺肿瘤细胞的自噬,进而促进肿瘤细胞迁移。然而,SEC23B如何抑制ERK/mTOR信号转导通路的机制以及SEC23B的上游信号转导通路仍需进一步探索。
综上所述,本研究发现SEC23B通过抑制ERK/mTOR信号转导通路来诱导乳腺癌细胞自噬,并促进肿瘤细胞转移。结果表明SEC23B可能是一种有应用前景的预后生物标志物和乳腺癌治疗的潜在治疗靶点。然而,由于本研究没有纳入临床病例分析SEC23B在乳腺癌组织中的表达情况,并且分析SEC23B生存数据时只是采用单因素分析,未来需要在临床队列中通过多因素模型来验证本研究的结果。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1] 王 帅, 崔中豪, 杨 毅. HER2阳性乳腺癌脑转移的靶向治疗研究进展[J]. 医学研究生学报, 2020, 33(2): 215-219.
WANG S, CUI Z H, YANG Y. Research progress of targeted therapy for HER2 positive breast cancer with brain metastases[J]. J Med Postgrad, 2020, 33(2): 215-219.
[2] FARES J, FARES M Y, KHACHFE H H, et al. Molecular principles of metastasis: a hallmark of cancer revisited[J]. Signal Transduct Target Ther, 2020, 5(1): 28.
[3] ARAKEL E C, SCHWAPPACH B. Formation of COPI-coated vesicles at a glance[J]. J Cell Sci, 2018, 131(5): jcs209890.
[4] WEI W, LIU Z G, ZHANG C, et al. A common human missense mutation of vesicle coat protein SEC23B leads to growth restriction and chronic pancreatitis in mice[J]. J Biol Chem, 2022, 298(1): 101536.
[5] ZHOU J G, SINGH P, YIN K H, et al. Non-medullary thyroid cancer susceptibility genes: evidence and disease spectrum[J]. Ann Surg Oncol, 2021, 28(11): 6590-6600.
[6] RAMALHO-CARVALHO J, MARTINS J B, CEKAITE L, et al. Epigenetic disruption of miR-130a promotes prostate cancer by targeting SEC23B and DEPDC1[J]. Cancer Lett, 2017, 385: 150-159.
[7] LIU Z, WANG Q, MAO J W, et al. Comparative proteomic analysis of protein methylation provides insight into the resistance of hepatocellular carcinoma to 5-fluorouracil[J]. J Proteomics, 2020, 219: 103738.
[8] ZEYEN L, DÖRING T, STIELER J T, et al. Hepatitis B subviral envelope particles use the COPII machinery for intracellular transport via selective exploitation of Sec24A and Sec23B[J]. Cell Microbiol, 2020, 22(6): e13181.
[9] 周 慧, 韩 翰, 周伟强. SAHA-CTSV轴通过诱导过度自噬抑制乳腺癌MCF-7细胞的生长[J]. 中国药理学通报, 2021, 37(4): 504-510.
ZHOU H, HAN H, ZHOU W Q. SAHA-CTSV induced excessive autophagy and inhibited growth of breast cancer MCF-7 cells[J]. Chin Pharmacol Bull, 2021, 37(4): 504-510.
[10] MAITI A, HAIT N C. Autophagy-mediated tumor cell survival and progression of breast cancer metastasis to the brain[J]. J Cancer, 2021, 12(4): 954-964.
[11] KING R, LIN Z S, BALBIN-CUESTA G, et al. SEC23A rescues SEC23B-deficient congenital dyserythropoietic anemia type Ⅱ[J]. Sci Adv, 2021, 7(48): eabj5293.
[12] YEHIA L, NIAZI F, NI Y, et al. Germline heterozygous variants in SEC23B are associated with cowden syndrome and enriched in apparently sporadic thyroid cancer[J]. Am J Hum Genet, 2015, 97(5): 661-676.
[13] YEHIA L, JINDAL S, KOMAR A A, et al. Non-canonical role of cancer-associated mutant SEC23B in the ribosome biogenesis pathway[J]. Hum Mol Genet, 2018, 27(18): 3154-3164.
[14] PIFFOUX M, ERIAU E, CASSIER P A. Autophagy as a therapeutic target in pancreatic cancer[J]. Br J Cancer, 2021, 124(2): 333-344.
[15] DOWER C M, WILLS C A, FRISCH S M, et al. Mechanisms and context underlying the role of autophagy in cancer metastasis[J]. Autophagy, 2018, 14(7): 1110-1128.
[16] FAN Q, YANG L, ZHANG X D, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells[J]. J Exp Clin Cancer Res, 2018, 37(1): 9.
[17] JEONG Y T, SIMONESCHI D, KEEGAN S, et al. The ULK1-FBXW5-SEC23B nexus controls autophagy[J]. Elife, 2018, 7: e42253.
[18] HUANG S F, TANG M Z, JIANG H L, et al. A COPII subunit interacting with ER-phagy receptor: a new potential avenue to maintaining neuronal homeostasis[J]. Acta Biochim Biophys Sin (Shanghai), 2020, 52(6): 698-700.
[19] XU H Y, LIU L Y, DING M, et al. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells[J]. Int J Biol Macromol, 2020, 146: 353-362.
[20] YANG C Y, CHEN N, LI X, et al. Mutations in the coat complex Ⅱ component SEC23B promote colorectal cancer metastasis[J]. Cell Death Dis, 2020, 11(3): 157.


