【论著】| 激素敏感性前列腺癌患者中PSMA PET/CT衍生参数与循环肿瘤DNA特征之间的相关性分析
时间:2024-08-25 08:03:14 热度:37.1℃ 作者:网络
[摘要] 背景与目的:前列腺特异性膜抗原(prostate-specific membrane antigen,PSMA)正电子发射计算机体层显像(positron emission tomography/computed tomography,PET/CT)和循环肿瘤DNA(circulating tumor DNA,ctDNA)的检测结果都是激素敏感性前列腺癌(hormone-sensitive prostate cancer,HSPC)治疗决策的参考依据。本研究旨在分析HSPC患者中PSMA PET/CT衍生参数与ctDNA特征之间的相关性。方法:回顾性纳入于复旦大学附属肿瘤医院就诊且接受PSMA PET/CT和ctDNA测序的间隔≤2周、有完整病历记录的HSPC患者。排除存在除前列腺癌外的活动性恶性肿瘤,以及组织学特征支持纯神经内分泌癌或小细胞癌诊断的患者。本研究经复旦大学附属肿瘤医院伦理委员会批注(伦理编号:1909207-12)。采用Spearman相关系数评价PSMA PET/CT衍生参数最大标准摄取值(maximum standardized uptake value,SUVmax)、总肿瘤体积(total tumor volume,TTV)、病灶摄取总量(total lesion uptake,TLU)与ctDNA分数(ctDNA%)之间的相关性。结果:共纳入60例HSPC患者,TP53(3.3%)、BRCA2(3.3%)和ATM(3.3%)是最常见的突变基因。在相关性分析中,ctDNA%与SUVmax有显著相关性(Spearman’s rho=0.272,P=0.036);ctDNA%与TLU(Spearman’s rho=0.160,P=0.222)和TTV(Spearman’s rho=0.162,P=0.215)无显著相关性。结论:SUVmax与ctDNA%之间有显著相关性,提示与无PSMA阳性病灶和低PSMA摄取病灶的患者相比,存在高PSMA摄取病灶的患者接受联合靶向治疗的概率增加,本研究结果有望作为制订个体化治疗方案的参考。
[关键词] 激素敏感性前列腺癌;前列腺特异性膜抗原;正电子发射计算机体层显像;循环肿瘤DNA;相关性分析
[Abstract] Background and purpose: Both prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and circulating tumor DNA (ctDNA) sequencing outcomes serve as references for therapeutic decision-making in hormone-sensitive prostate cancer (HSPC) treatment. This study aimed to analyze the association between PSMA PET/CT-derived parameters and ctDNA characteristics in patients with HSPC. Methods: HSPC patients who received PSMA PET/CT and ctDNA sequencing at an interval of less than 2 weeks and with complete medical records were retrospectively included in Fudan University Shanghai Cancer Center. Patients with active malignancies other than prostate cancer and those with histological features supporting a diagnosis of pure neuroendocrine carcinoma or small cell carcinoma were excluded. This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (Ethics number: 1909207-12). The correlation between PSMA PET/CT-derived parameters, including the maximum standardized uptake value (SUVmax), total tumor volume (TTV), total lesion uptake (TLU) and ctDNA fraction (ctDNA%) was evaluated using the Spearman correlation coefficient. Results: A total of 60 HSPC patients were included, with TP53 (3.3%), BRCA2 (3.3%) and ATM (3.3%) being the most common mutated genes. In the correlation analysis, a significant correlation was observed between ctDNA% and SUVmax levels (Spearman’s rho=0.272, P=0.036); however, no significant correlation was found between ctDNA% and TLU (Spearman’s rho=0.160, P=0.222) or TTV (Spearman’s rho=0.162, P=0.215). Conclusion: There was a significant correlation between SUVmax and ctDNA%, suggesting that patients with high PSMA uptake lesions were more likely to receive combined targeted therapy than patients with no PSMA positive lesions and patients with low PSMA uptake lesions, which provided a certain reference for the formulation of individualized treatment plans.
[Key words] Hormone-sensitive prostate cancer; Prostate-specific membrane antigen; Positron emission tomography/computed tomography; Circulating tumor DNA; Correlation analysis
前列腺癌是全球男性的第二大常见恶性肿瘤[1-2]。虽然局限性前列腺癌患者的5年生存率接近100%,但转移性前列腺癌目前仍是难以治愈的[3-5]。因此通过影像学检查确定前列腺癌扩散的程度对于激素敏感性前列腺癌(hormone-sensitive prostate cancer,HSPC)患者的治疗决策非常重要。前列腺特异性膜抗原(prostate-specific membrane antigen,PSMA)正电子发射计算机体层显像(positron emission tomography/computed tomography,PET/CT)是前列腺癌的主要诊断方法之一,与其他分子影像技术(包括18F-fluoromethylcholine PET[6]、18F-fluciclovine PET[7]及传统影像学技术[8])相比显示出更高的诊断准确性。PSMA PET/CT可以提供准确的疾病分期并显著改善HSPC的治疗效果,通过改进的靶区描绘,可以更有效地通过影像引导治疗[9-11]。
基于血浆中循环肿瘤DNA(circulating tumor DNA,ctDNA)的高通量测序技术,能反映来源于前列腺癌细胞相关的遗传学特征,如扩增、甲基化、重排或基因突变等,可以作为癌症预防筛查、伴随诊断、疗效评估及预后风险分层的重要指标[12-13]。因其微创、检测所需时间短、能够反映瘤内和转移灶异质性、可动态监测疗效等优势而在前列腺癌精准诊疗中得到越来越广泛的应用[13]。
PSMA PET/CT衍生参数最大标准摄取值(maximum standardized uptake value,SUVmax)、总肿瘤体积(total tumor volume,TTV)及病灶摄取总量(total lesion uptake,TLU)不仅是前列腺癌的预后标志物,也是重要的治疗选择标准[14-16]。ctDNA分数(ctDNA%)同样不仅可用于指导用药,也与治疗预后相关[15]。因此,本研究旨在分析PSMA PET/CT衍生参数与ctDNA特征之间的相关性,以深入了解HSPC的生物学特性,并为实现更加个性化的治疗方案提供参考数据。
1 资料和方法
1.1 研究对象
回顾性纳入于复旦大学附属肿瘤医院就诊的HSPC患者。纳入标准包括:① 根据前列腺癌临床试验工作组3(Prostate Cancer Clinical Trials Working Group 3,PCWG3)标准[17]确诊为HSPC;② 接受PSMA PET/CT和ctDNA测序的间隔≤2周;③ 有完整的病历记录。排除标准包括:① 存在除前列腺癌外的活动性恶性肿瘤;② 组织学特征支持纯神经内分泌癌或小细胞癌诊断的患者。本研究经复旦大学附属肿瘤医院伦理委员会批准(伦理编号:1909207-12)。
1.2 PSMA PET/CT参数解析
所有的PSMA PET/CT都遵循标准操作程序[14-16,18]。2名在PSMA PET/CT阅读方面具有超过5年经验的核医学科医师,在对患者的组织病理学/活检结果和后续的影像学检查结果均不知情的情况下,采用视觉分析并对感兴趣区(region of interest,ROI)行半定量分析,对结果进行独立审查和解读。当意见不一致时,2名核医学科医师需要经过讨论后达成共识。PSMA阳性病灶被定义为摄取在局部凝聚并且高于血池(肝脏血池)。SUVmean为ROI中SUV的平均值。对于每个病例,获得SUVmax、TTV和TLU。TTV的计算公式为:TTV=∑TV;TLU的计算公式为:TLU=∑TV×SUVmean[18]。
1.3 ctDNA测序
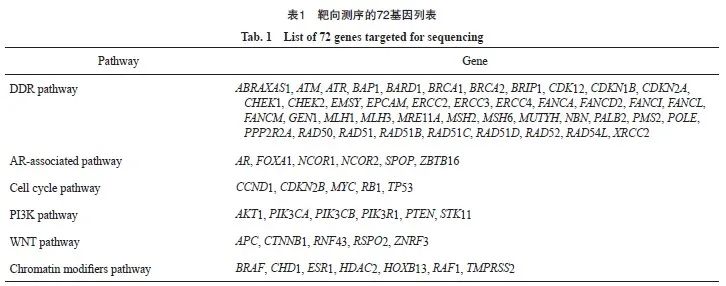
DNA提取、靶向捕获测序和生物信息学分析的详细方法参考之前发表的研究成果[15-16],采用包含72个基因的ProstatePlasma Panel(燃石医学有限责任公司)进行ctDNA的靶向测序(表1)。在Nextseq 500测序仪(美国Illumina公司)上进行成对末端测序,具有成对末端读取和平均目标测序深度为10 000X的特点。在序列数据分析中,检测到了体细胞突变和拷贝数变化。每个样本的ctDNA%定义为检测到的ctDNA突变的最大变异等位基因频率。胚系突变被排除在后续分析之外。ctDNA检出率定义为检测到致病性体细胞突变的患者比例[15-16]。
1.4 统计学处理
采用SPSS 22.0软件对数据进行统计学分析。采用中位数和四分位距(interquartile range,IQR)来表示连续性变量,频率和百分比来表示分类变量。采用Spearman相关系数评价连续性变量(SUVmax、TTV和TLU)与ctDNA%之间的相关性[16]。P<0.05为差异有统计学意义,检验均为双侧。
2 结 果
2.1 基线特征
共纳入60例HSPC患者。中位年龄为65岁(IQR:57~75岁),中位前列腺特异性抗原(prostate-specific antigen,PSA)水平为0.14 ng/ mL (IQR:0.01~7.26 ng/mL)。值得注意的是,41例(68.3%)患者的Gleason评分>7分,17例(28.3%)患者的Gleason评分≤7分,2例(3.3%)患者因在雄激素剥夺治疗(androgen deprivation therapy,ADT)后行穿刺而无Gleason评分。转移的主要部位是淋巴结(24例,40.0%;其中远处淋巴结转移13例),其次是骨骼(22例,36.7%)和肺(2例,3.3%)。
60例患者中,13例(21.7%)存在体细胞突变(图1)。TP53(3.3%)、BRCA2(3.3%)和ATM(3.3%)是最常见的突变基因,其次是ERCC4(1.7%)、MSH6(1.7%)、ATR(1.7%)、SPOP(1.7%)、CDKN2A (1.7%)、CHD1(1.7%)、FOXA1(1.7%)、CDK12(1.7%)、PIK3CA(1.7%)、CHEK2 (1.7%)和NCOR2(1.7%)。3例(5.0%)患者存在2个突变,其余患者均只存在1个突变。
2.2 相关性分析
在可检出ctDNA的患者中,ctDNA%的中位数为4.1%,最高为51.1%。总体上,中位TLU和TTV分别为15.37和5.97。在相关性探索分析中,ctDNA%与SUVmax有显著相关性(Spearman’s rho=0.272,P=0.036,图2A),ctDNA%与TLU(Spearman’s rho=0.160,P=0.222,图2B)和TTV(Spearman’s rho=0.162,P=0.215,图2C)无显著相关性。
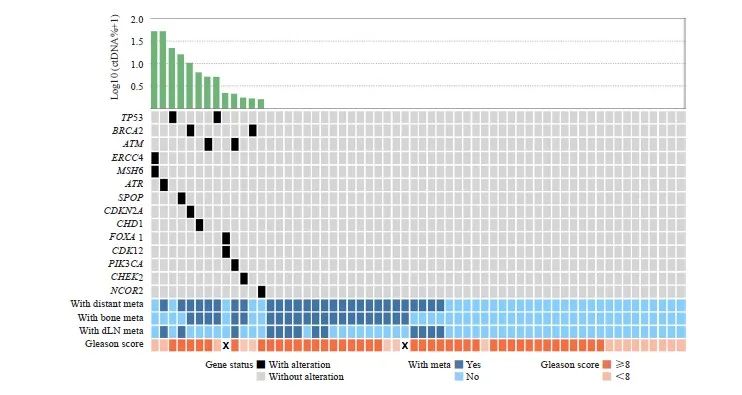
图1 60例HSPC患者的突变谱
Fig. 1 Mutation landscape of 60 patients with HSPC
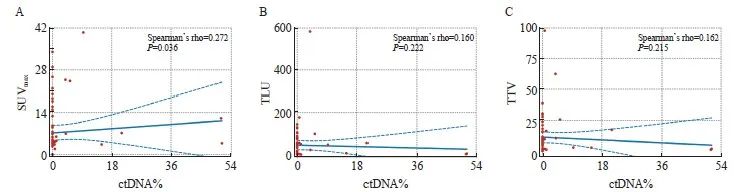
图2 SUVmax(A)、TLU(B)和TTV(C)与ctDNA%之间的相关性分析
Fig. 2 Correlation analysis of SUVmax (A), TLU (B) and TTV (C) with ctDNA%
3 讨 论
在癌症的常规管理中,疾病的动态监测十分重要。尽管前列腺癌的治疗手段取得了重大进展,但其治疗监测仍然是一项迫切需要解决的问题。因此,PSMA PET/CT检查和CPRC患者的ctDNA检查的使用不断增加,通过实时的分子和影像特征,可以对患者进行分层管理,从而作出适宜的治疗决策[16,18-19]。在HSPC中,尚未见关于这两种检测的参数相关性的报道。本研究分析了PSMA PET/CT衍生参数与ctDNA特征之间的相关性,发现SUVmax与ctDNA%有显著相关性。
PSMA PET/CT不仅是前列腺癌的成像手段,基于PSMA的放射性配体疗法的研究也越来越多[2]。2022年,美国食品药品管理局(Food and Drug Administration,FDA)批准177Lu-PSMA-617用于治疗已接受雄激素受体途径抑制剂和紫杉烷类药物化疗且在PSMA PET/CT上存在阳性病灶的转移性去势抵抗性前列腺癌患者。对于177Lu-PSMA-617治疗,最重要的筛选指标是PSMA PET/CT衍生参数SUVmax[20-21]。在TheraP试验[21]中,177Lu-PSMA-617治疗组患者需存在至少1个SUVmax>20的病灶,且其余可测量病灶的SUVmax>10。VISION临床试验[20]则要求患者至少存在1个PSMA PET/CT阳性病灶。目前探索177Lu-PSMA-617联合标准治疗的临床试验也逐渐在各阶段前列腺癌中开展,包括HSPC[22]。例如,Eapen等[22]在一项单臂、Ⅰ/Ⅱ期临床试验中,发现在高危局限性前列腺癌患者的根治性前列腺切除术之前给予最多2个周期的177Lu-PSMA-617是安全可行的。本研究显示,SUVmax与ctDNA%之间有显著相关性,提示与无PSMA阳性病灶和低PSMA摄取病灶的患者相比,存在高PSMA摄取病灶的患者接受联合靶向治疗的概率增加[15]。基于此发现,后续在前瞻性队列研究中根据不同肿瘤负荷或摄取强度进行选择性的基因检测,可能是未来探索的一个方向。例如,针对高于摄取阈值或VISION临床试验[20]筛选标准的HSCP患者,推荐进行ctDNA的靶向深度测序。
40%~70%的中国初诊前列腺癌患者已处于转移阶段,而前列腺癌发生、发展的时空异质性及独特的转移模式使基于活检组织取材分析生物标志物的方式困难重重[2]。因此,作为一种能反映全身大多数肿瘤组织中发现的体细胞变异的微创生物标志物,ctDNA具有广泛的应用前景[2,13,23]。ctDNA脱落主要是由肿瘤细胞凋亡和坏死引起的[24]。通过ctDNA检测分析突变和拷贝数变化,可对HSPC肿瘤的基因组亚型进行分类。本研究中,因大部分HSPC处于ADT应答之中,HSPC患者的PSA水平较低(中位数为 0.14 ng/mL),即使是在高测序深度下,ctDNA检出率也较低[15,25]。低ctDNA检出率同时限制了单个基因和通路的富集分析[16]。随着技术发展,cfDNA的检测灵敏度会增加,低PSA水平的患者也可从ctDNA检测中得到治疗提示[13]。与文献报道[16]中去势抵抗性前列腺癌中的分析结果不同,HSPC中的ctDNA%与肿瘤负荷(包括TTV和TLU)之间并不存在相关性。产生这种差异的原因可能是去势抵抗性前列腺癌患者中存在高骨转移率(56%)和内脏转移率(20%)[16],而本研究的HSPC队列中仅为37%和3%。此外,SUVmax为全身PSMA摄取最高病灶的量化,其与有意义的突变检出之间的关联,提示影响分子突变图谱的最大变量可能是恶性程度最高的病灶。其他潜在的影响因素,例如,肿瘤生物学特异性脱落动力学和清除率也可能影响ctDNA与肿瘤负荷之间的相关性[26-27],特别是在低容量病例中,但这无法通过本次小规模单中心队列研究证实。
本研究仍存在一定局限性。首先,单中心的数据不可避免地有人群选择性偏倚,但非选择性方式降低了偏倚带来的误差;其次,有限的样本数一定程度上会影响统计学分析结果的准确性,将来我们会在更大样本量、更均质的队列中进行多中心、随机的前瞻性试验来验证本研究的结论;最后,由于随访时间有限,本研究没有展示联合分子分型与PSMA PET/CT参数的HSPC预后模型。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
潘剑:实施研究,收集并分析数据,撰写并审阅文章。
叶定伟,朱耀,王备合:构思研究,审阅文章。
[参考文献]
[1]DÖRR M, HÖLZEL D, SCHUBERT-FRITSCHLE G, et al. Changes in prognostic and therapeutic parameters in prostate cancer from an epidemiological view over 20 years[J]. Oncol Res Treat, 2015, 38(1/2): 8-14.
[2]潘 剑, 朱 耀, 戴 波, 等. 2022年度前列腺癌基础研究及临床诊疗新进展[J]. 中国癌症杂志, 2023, 33(3): 210-217.
PAN J, ZHU Y, DAI B, et al. Advances in basic research, clinical diagnosis and treatment of prostate cancer in 2022[J]. China Oncol, 2023, 33(3): 210-217.
[3]WU J N, FISH K M, EVANS C P, et al. No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period[J]. Cancer, 2014, 120(6): 818-823.
[4]CRAWFORD E D, PETRYLAK D, SARTOR O. Navigating the evolving therapeutic landscape in advanced prostate cancer[J]. Urol Oncol, 2017, 35S: S1-S13.
[5]PAN J, YE D W, ZHU Y. Olaparib outcomes in metastatic castration-resistant prostate cancer: first real-world experience in safety and efficacy from the Chinese mainland[J]. Prostate Int, 2022, 10(3): 142-147.
[6] EMMETT L, METSER U, BAUMAN G, et al. Prospective, multisite, international comparison of 18F-fluoromethylcholine PET/CT, multiparametric MRI, and 68Ga-HBED-CC PSMA-11 PET/CT in men with high-risk features and biochemical failure after radical prostatectomy: clinical performance and patient outcomes[J]. J Nucl Med, 2019, 60(6): 794-800.
[7] CALAIS J, CECI F, EIBER M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial[J]. Lancet Oncol, 2019, 20(9): 1286-1294.
[8] HOFMAN M S, LAWRENTSCHUK N, FRANCIS R J, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study[J]. Lancet, 2020, 395(10231): 1208-1216.
[9] CALAIS J, FENDLER W P, EIBER M, et al. Impact of 68Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence[J]. J Nucl Med, 2018, 59(3): 434-441.
[10] PIENTA K J, GORIN M A, ROWE S P, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY)[J]. J Urol, 2021, 206(1): 52-61.
[11] HADASCHIK B, OST P. Re: assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial[J]. Eur Urol, 2019, 76(4): 538-539.
[12] PASCUAL J, ATTARD G, BIDARD F C, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group[J]. Ann Oncol, 2022, 33(8): 750-768.
[13] SHEN H R, LIU J L, CHEN K X, et al. Language model enables end-to-end accurate detection of cancer from cell-free DNA[J]. Brief Bioinform, 2024, 25(2): bbae053.
[14] PAN J, WEI Y, ZHANG T W, et al. Stereotactic radiotherapy for lesions detected via 68Ga-prostate-specific membrane antigen and 18F-fluorodexyglucose positron emission tomography/ computed tomography in patients with nonmetastatic prostate cancer with early prostate-specific antigen progression on androgen deprivation therapy: a prospective single-center study[J]. Eur Urol Oncol, 2022, 5(4): 420-427.
[15] PAN J, ZHAO J O, NI X D, et al. The prevalence and prognosis of next-generation therapeutic targets in metastatic castrationresistant prostate cancer[J]. Mol Oncol, 2022, 16(22): 4011-4022.
[16] PAN J, ZHAO J O, NI X D, et al. Heterogeneity of[68Ga]Ga- PSMA-11 PET/CT in metastatic castration-resistant prostate cancer: genomic characteristics and association with abiraterone response[J]. Eur J Nucl Med Mol Imaging, 2023, 50(6): 1822-1832.
[17] SCHER H I, MORRIS M J, STADLER W M, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3[J]. J Clin Oncol, 2016, 34(12): 1402-1418.
[18] PAN J, ZHANG T W, CHEN S Z, et al. Nomogram to predict the presence of PSMA-negative but FDG-positive lesion in castration-resistant prostate cancer: a multicenter cohort study[J]. Ther Adv Med Oncol, 2024, 16: 17588359231220506.
[19] PAN J, WANG J, WEI Y, et al. Combination of body mass index and albumin predicts the survival in metastatic castrationresistant prostate cancer patients treated with abiraterone: a post hoc analysis of two randomized trials[J]. Cancer Med, 2021, 10(19): 6697-6704.
[20] SARTOR O, BONO J D, CHI K N, et al. Lutetium-177- PSMA-617 for metastatic castration-resistant prostate cancer[J]. N Engl J Med, 2021, 385(12): 1091-1103.
[21] HOFMAN M S, EMMETT L, SANDHU S, et al. [177Lu] Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial[J]. Lancet, 2021, 397(10276): 797-804.
[22] EAPEN R S, BUTEAU J P, JACKSON P, et al. Administering[177Lu]Lu-PSMA-617 prior to radical prostatectomy in men with high-risk localised prostate cancer (LuTectomy): a singlecentre, single-arm, phase 1/2 study[J]. Eur Urol, 2024, 85(3): 217-226.
[23] COHEN S A, LIU M C, ALESHIN A. Practical recommendations for using ctDNA in clinical decision making[J]. Nature, 2023, 619(7969): 259-268.
[24] JAHR S, HENTZE H, ENGLISCH S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells[J]. Cancer Res, 2001, 61(4): 1659-1665.
[25] VANDEKERKHOVE G, STRUSS W J, ANNALA M, et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer[J]. Eur Urol, 2019, 75(4): 667-675.
[26] CORCORAN R B, CHABNER B A. Application of cell-free DNA analysis to cancer treatment[J]. N Engl J Med, 2018, 379(18): 1754-1765.
[27] VOLIK S, ALCAIDE M, MORIN R D, et al. Cell-free DNA (cfDNA): clinical significance and utility in cancer shaped by emerging technologies[J]. Mol Cancer Res, 2016, 14(10): 898-908.


