【论著】| M1型肿瘤相关巨噬细胞在肝细胞癌组织中浸润的意义
时间:2024-09-26 15:00:21 热度:37.1℃ 作者:网络
[摘要] 背景与目的:肿瘤相关巨噬细胞(tumor-associated macrophages,TAM)是肿瘤微环境中的主要基质细胞,在肿瘤进展过程中发挥重要作用,本研究旨在探究肝细胞癌(hepatocellular carcinoma,HCC)中M1型TAM浸润的临床意义。方法:收集2012年1月—2020年12月在南通大学附属南通第三医院接受手术的HCC患者石蜡包埋组织样本320例,采用免疫组织化学法检测CD86标记的M1型TAM在HCC组织中分布情况,计算阳性细胞密度,根据细胞密度分组:大于平均密度(29个/mm2)判定为高密度组,小于或等于平均密度为低密度组;统计分析M1型TAM密度与HCC临床病理学特征、肿瘤浸润CD8+T淋巴细胞之间的相关性及预后意义;采用免疫组织化学法检测程序性死亡配体-1(programmed death ligand-1, PD-L1)的表达情况,根据CD86、PD-L1细胞密度将病例分4组:CD86+高密度组中PD-L1高密度(CD86highPD-L1high)和PD-L1低密度(CD86highPD-L1low)组;CD86+低密度组中PD-L1高密度(CD86lowPD-L1high)和PD-L1低密度(CD86lowPD-L1low)组,分析CD86+ M1型TAM密度联合PD-L1表达的预后意义。本研究通过南通大学附属南通第三医院伦理委员会批准(伦理编号:EK2022005)。结果:CD86+M1型TAM主要分布于肿瘤间质中;其高密度率为44.7%(143/320)。CD86+M1型TAM密度与CD8+肿瘤浸润细胞毒性T淋巴细胞密度呈正相关(P<0.001)、与乙型肝炎病毒表面抗原(hepatitis B virus surface antigen,HBsAg)阳性呈负相关(P=0.003),与患者性别、年龄、肝硬化、肿瘤大小、组织学分级、微血管侵犯等临床病理学指标均无明显相关性;CD86+M1型TAM高密度组患者总生存期(overall survival,OS)、无病生存期(disease-free survival,DFS)优于低密度组,差异均有统计学意义(P均<0.001)。多因素Cox比例风险回归模型分析显示,低密度CD86+M1型TAM是评估OS和DFS的独立风险因子(OS:HR=1.468,P=0.022;DFS:HR=2.233,P<0.001)。CD86highPD-L1high组HCC患者OS、DFS差于CD86highPD-L1low组,两者差异有统计学意义(P均<0.05)。 CD86lowPD-L1high组OS、DFS差于CD86lowPD-L1low组,两者OS差异有统计学意义(P<0.05),DFS差异无统计学意义。结论:HCC组织中存在高密度CD86+M1型TAM提示患者预后良好,并且是独立的预后因子。HCC组织表达PD-L1提示肿瘤侵袭性增强,患者预后差。
[关键词] 巨噬细胞;程序性死亡配体1;肝细胞癌;预后
[Abstract] Background and purpose: Tumor-associated macrophages (TAM) as the main stromal cells in the tumor microenvironment play an important role in tumor progression. This study aimed to explore the clinical significance of M1 type TAM infiltration in hepatocellular carcinoma (HCC). Methods: We collected tissue paraffin samples from 320 HCC patients who underwent surgery at the Affiliated Nantong Hospital Three of Nantong University from January 2012 to December 2020. Immunohistochemical methods were used to detect the distribution of CD86 labeled M1 type TAM in HCC tissues, and positive cell density was calculated. Groups were established according to cell density, high-density group had cells with greater than average density (29 cells/mm2), and low-density group had cells with less than or equal to average density. The correlation and prognostic significance of M1 TAM density with clinicopathologic features and tumor infiltrating CD8+ T lymphocytes of HCC were analyzed. Using immunohistochemistry to detect the expression of programmed death ligand-1 (PD-L1), the cases were divided into four groups based on the cell density of CD86 and PD-L1. In the CD86+ high-density group, PD-L1 high-density (CD86highPD-L1low) and PD-L1 low-density (CD86highPD-L1low) groups were included. In the CD86+ low-density group, the PD-L1 high-density (CD86lowPD-L1high) and PD-L1 low-density (CD86lowPD-L1low) groups were included. We analyzed the prognostic significance of CD86+ M1 type TAM density combined with PD-L1 expression. This study was approved by the Ethics Committee of Affiliated Nantong Hospital Three of Nantong University (ethics number: EK2022005). Results: CD86+ M1 type TAM was mainly distributed in the tumor stroma. Its high-density rate was 44.7% (143/320). The density of CD86+ M1 type TAM was positively correlated with tumor infiltrating CD8+ T lymphocyte density (P<0.001) and negatively correlated with hepatitis B virus surface antigen (HBsAg) positivity (P=0.003), and had no significant correlation with clinical and pathological features such as patient age, gender, cirrhosis, tumor size, histological grading and microvascular invasion. The CD86+ M1 type TAM high-density group had better overall survival (OS) and disease-free survival (DFS) than the low-density group, and the differences were statistically significant (all P<0.001). Multivariate Cox proportional hazards regression model analysis showed that low-density CD86+ M1 type TAM was an independent risk factor for evaluating OS and DFS (OS: HR=1.468, P=0.022; DFS: HR=2.233, P<0.001). The CD86highPD-L1high group had poor OS and DFS than the CD86highPD-L1low group, and the differences were statistically significant (both P<0.05). The CD86lowPD-L1high group had poor OS and DFS than the CD86lowPD-L1low group. The difference in OS between the two groups was statistically significant (P<0.05), while the difference in DFS was not statistically significant. Conclusion: The presence of high-density CD86+ M1 type TAM in HCC tissue suggests a good prognosis and is an independent prognostic factor. Expression of PD-L1 in HCC tissue suggests increased invasiveness and poorer prognosis.
[Key words] Macrophage; Programmed death ligand 1; Hepatocellular carcinoma; Prognosis
肝细胞癌(hepatocellular carcinoma,HCC)是一种与免疫耐受和免疫微环境逃避有关的炎症相关肿瘤。免疫治疗可以打破免疫耐受,提高机体的免疫反应,进而识别并杀死肿瘤细胞。肿瘤微环境中M1和M2型肿瘤相关巨噬细胞(tumor-associated macrophages,TAM)的极化稳态与肿瘤的发生、发展有关,一直是肿瘤研究的热点[1-2]。程序性死亡配体-1(programmed death ligand-1,PD-L1)在TAM的极化中起着重要作用,并作为免疫治疗的靶点影响HCC患者的预后。之前本研究小组已报道M2型TAM在HCC中浸润的临床意义[3],本研究进一步探讨M1型巨噬细胞在HCC中的分布状态、临床病理学意义及预后评估中的应用价值,并联合PD-L1表达情况分析其对患者预后的意义。
1 资料和方法
1.1 病历资料
选取病例为2012年1月—2020年12月在南通大学附属南通第三医院接受手术的患者。纳入标准:① 首次肝肿瘤根治性切除;② 经病理学检查证实为HCC;③ 完整的临床数据;④ 没有癌症治疗史。排除标准:① 接受姑息性手术或无法完成切除的患者;② 不完整的临床数据;③ 接受过靶向或免疫等治疗。共纳入320例患者,其中男性252例,女性68例;平均年龄54.9岁。乙型肝炎病毒表面抗原(hepatitis B virus surface antigen,HBsAg)采用化学发光法检测患者血清学样本,阳性260例。320例患者末次随访时间为2022年12月31日,中位随访时间为32个月。至末次随访时,183例出现复发,162例死亡。本研究通过南通大学附属南通第三医院伦理委员会批准(伦理编号:EK2022005),所有患者均签署知情同意书。
1.2 免疫组织化学法检测
本研究采用4%的甲醛溶液固定石蜡包埋组织切片脱蜡水化,随后置于乙二胺四乙酸(ethylenediaminetetraacetic acid,EDTA)溶液(pH=9.0)中进行高温抗原修复;之后将切片置于H2O2溶液中温育15 min。PBS冲洗后加一抗,分别为:小鼠抗CD86单克隆抗体(克隆号C86/1146;1∶200)购自英国Abcam公司、兔抗PD-L1单克隆抗体(克隆号ZR3;1∶100)购自基因科技(上海)股份有限公司,25 ℃温育2 h。用辣根过氧化物酶(horseradish peroxidase,HRP)标记的二抗25 ℃温育30 min。最后用二氨基联苯胺(diaminobenzidine,DAB)显色。
1.3 结果判定
CD86阳性染色判断标准为:免疫组织化学结果显示棕黄色信号定位于细胞质(不论颜色深浅)判定为CD86阳性。PD-L1阳性染色判断标准为:棕黄色信号定位于细胞膜或细胞膜和细胞质判定为PD-L1阳性。
采用如下方法进行阳性细胞计数:在400×放大倍数(0.237 mm2/视野)下测量整张切片肿瘤巢内和肿瘤间质的相应面积,然后计数每个视野CD86阳性细胞数量。将阳性细胞总数除以面积得到细胞密度,计算平均细胞密度。>平均细胞密度判为高密度组,≤平均细胞密度判为低密度组。采用同样方法计数和判定PD-L1阳性细胞密度分组。
由两位具有高级职称病理科医师通过双盲法评估;不一致病例进一步讨论,直到达成共识。
1.4 统计学处理
等级资料相关性分析采用Spearman相关分析。生存分析采用Kaplan-Meier方法,运用log-rank检验进行组间比较。采用Cox比例风险模型进行单因素和多因素回归分析,先进行单因素回归分析,筛选出与生存和复发有显著关系的变量,将筛选出的变量纳入多因素回归分析。P<0.05为差异有统计学意义。统计分析采用SPSS 22.0软件进行。
2 结 果
2.1 CD86阳性TAM在HCC肿瘤微环境中的分布情况
免疫组织化学结果显示,HCC肿瘤微环境中存在CD86+TAM,主要分布于肿瘤边缘间质中(图1A、B、E、F)。根据CD86+M1型TAM密度的平均值(29个/mm2)将其分为高密度组和低密度组,CD86的高密度率为44.7%(143/320)。
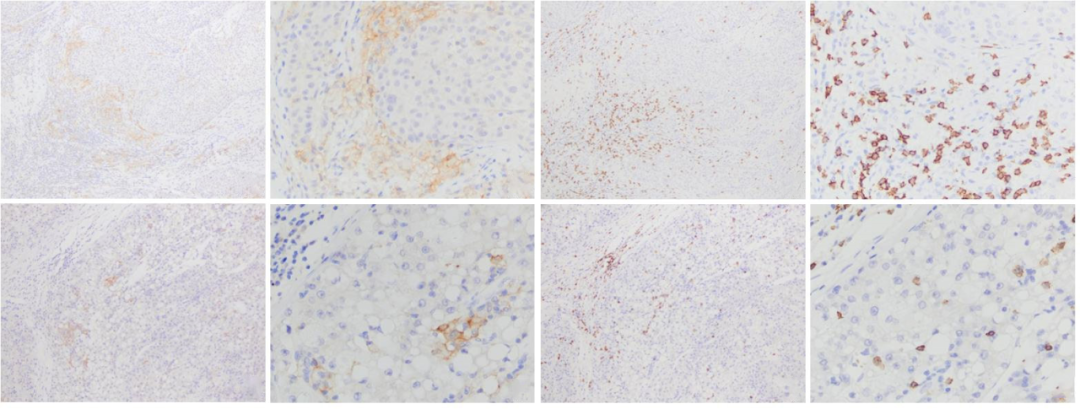
图1 免疫组织化学法检测HCC组织中CD86、CD8的表达情况
Fig. 1 Expressions of CD86 and CD8 in HCC tissues detected by immunohistochemistry
A, B: CD86 was highly density expresseed, B showed a partial magnification of A; C, D: CD8 labeled tumor-infiltrating T lymphocytes showed high density, D showed a partial magnification of C; A, B, C, and D displayed the same area of the same case; E, F: CD86 was lowly density expressed, F showed a partial magnification of E; G, H: CD8 labeled tumor-infiltrating T lymphocytes showed low density, H showed a partial magnification of G; E, F, G, and H displayed the same area of the same case. Envision staining; A, C, E, G (×100); B, D, F, H (×400).
2.2 CD86+M1型TAM的密度与HCC患者临床病理学特征的关系
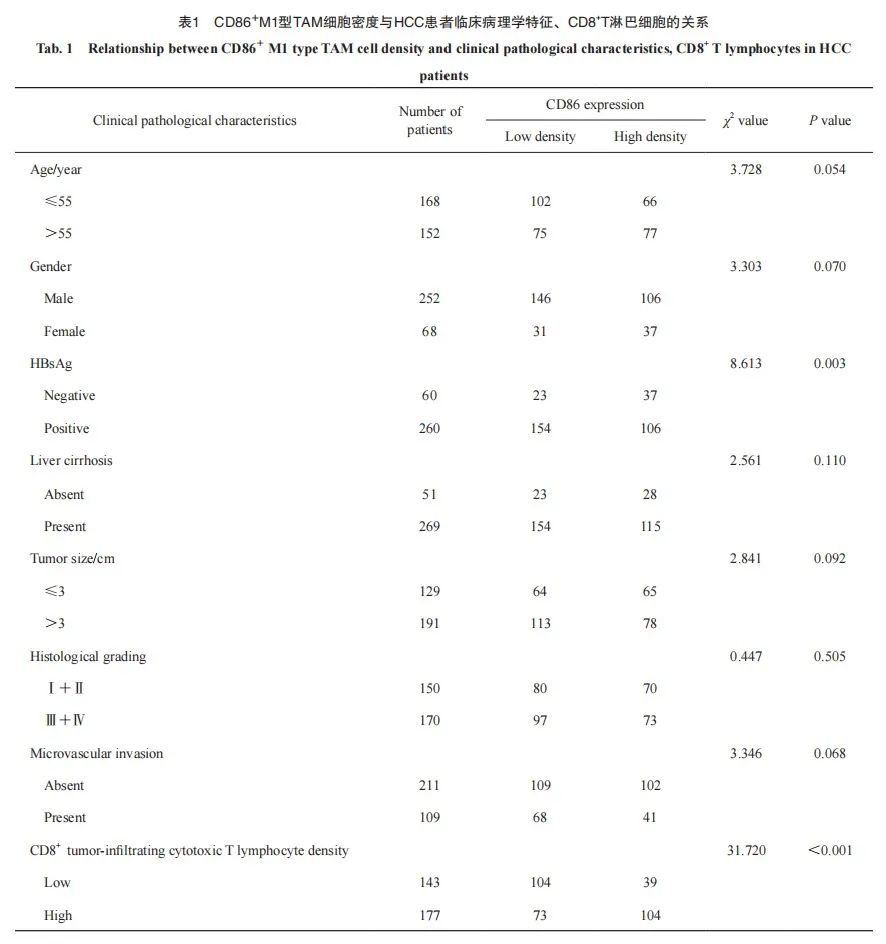
HCC组织中CD86+M1型TAM密度与肿瘤浸润CD8+T淋巴细胞密度呈正相关(x2=31.720,P<0.001,图1C、D、G、H),与HBsAg阳性呈负相关(x2=8.613,P=0.003);与患者性别、年龄、肿瘤大小、肝硬化、组织学分级、微血管侵犯等临床病理学指标均无明显相关性(表1)。
2.3 CD86+M1型TAM的密度与HCC患者预后的关系
CD86+M1型TAM高密度组HCC患者中位总生存期(overall survival,OS)为103个月,低密度组为38个月,两者预后差异有统计学意义(x2=15.037,P<0.001);CD86+M1型TAM高密度组HCC患者中位无病生存期(disease-free survival,DFS)为48个月,低密度组为26个月,两者预后差异有统计学意义(x2=17.145,P<0.001,图2)。
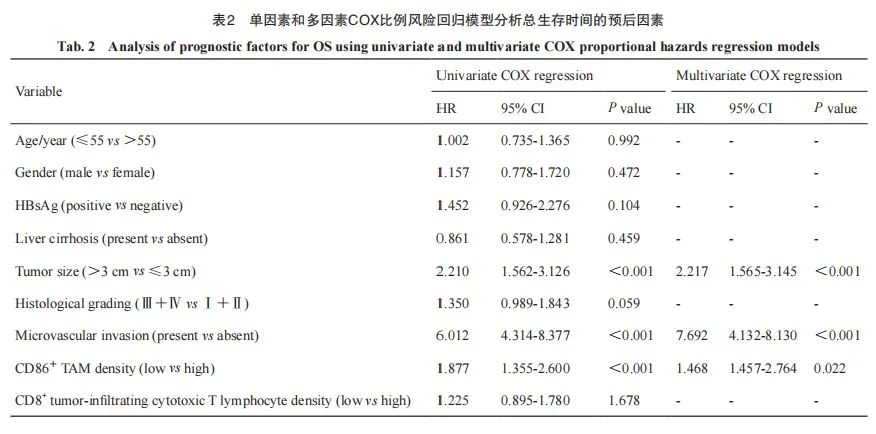
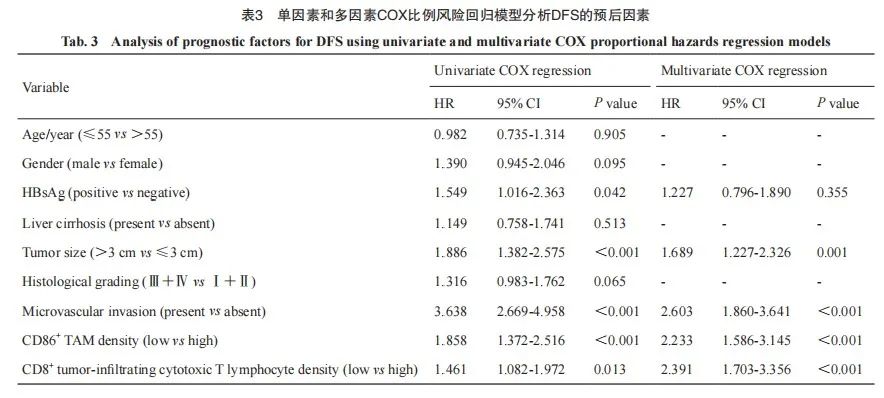
单因素Cox比例风险回归分析显示, HCC组织中CD86+M1型TAM低密度是影响OS和PFS的危险因素[OS:风险比(hazard ratio,HR)=1.877(1.355~2.600),P<0.001;DFS:HR=1.858(1.372~2.516),P<0.001]。同时,本组数据中肿瘤>3 cm[HR=2.210(1.562~3.126),P<0.001]、有微血管侵犯[HR=6.012(4.314~8.377),P<0.001]均是影响OS的危险因素。HBsAg阳性[HR=1.549(1.016~2.363),P=0.042]、肿瘤>3 cm[HR=1.886(1.382~2.575),P <0.001]、有微血管侵犯[HR=3.638(2.669~4.958),P<0.001]、肿瘤浸润CD86+T淋巴细胞低密度[HR=1.461(1.082~1.972),P=0.013]均是影响DFS的危险因素;将单因素Cox比例风险分析中的危险因素纳入多因素Cox比例风险回归分析,结果显示低密度CD86+M1型TAM是评估OS和DFS的独立危险因素[OS:HR=1.468(1.457~2.764),P=0.022;DFS:HR=2.233(1.586~3.145),P<0.001;表2、3]。
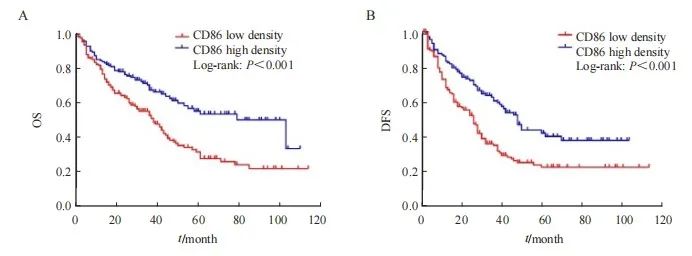
图2 基于CD86+M1型TAM密度的HCC患者生存率Kaplan-Meier曲线
Fig. 2 Kaplan-Meier curve for survival rate of HCC patients based on CD86+M1 type TAM density
A: The OS of CD86 high density group was higher than that of low density group; B: The DFS of CD86 high density group was higher than that of low density group.
2.4 CD86+M1型TAM密度联合PD-L1表达对HCC患者预后的影响
为了进一步分析CD86+M1型TAM密度联合PD-L1表达的预后意义,本研究将病例分4组比较:CD86+高密度组中PD-L1高密度(CD86highPD-L1high)和PD-L1低密度(CD86highPD-L1low)组;CD86+低密度组中PD-L1高密度(CD86lowPD-L1high)和PD-L1低密度(CD86lowPD-L1low)组。结果显示,CD86highPD-L1high组HCC患者中位OS、DFS为44和36个月,CD86highPD-L1low组为103和50个月,两者预后差异有统计学意义(OS:x2=9.915,P=0.002;DFS:x2=5.844,P=0.016;图3A、B)。CD86lowPD-L1high组中位OS、DFS为49和40个月,CD86lowPD-L1low组为103和62个月,两者OS差异有统计学意义(x2=6.502,P=0.011),DFS差异无统计学意义(x2=2.989,P=0.084;图3C、D)。
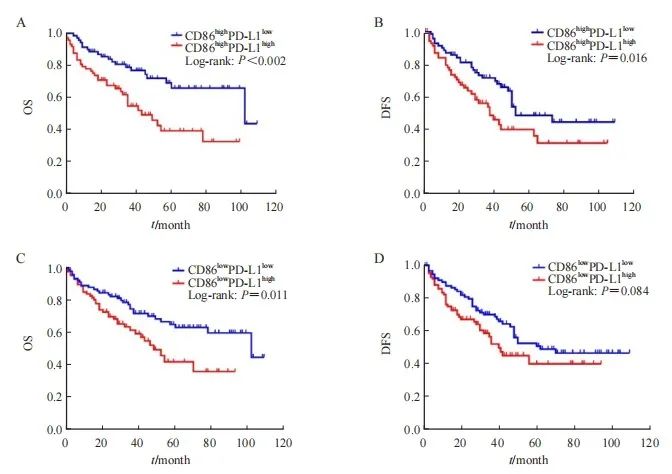
图3 基于CD86+M1型TAM密度联合PD-L1表达的HCC患者生存率Kaplan-Meier曲线
Fig. 3 Kaplan-Meier curve for survival rate of HCC patients based on CD86+ M1 type TAM density combined with PD-L1 expression
A: The OS of CD86highPD-L1high group was lower than that of CD86highPD-L1low group; B: The DFS of CD86highPD-L1high group was lower than that of CD86highPD-L1low group; C: The OS of CD86lowPD-L1high group was lower than that of CD86lowPD-L1low group; D: The DFS of CD86lowPD-L1high group was lower than that of CD86lowPD-L1low group, but the difference was not statistically significant (P=0.084).
3 讨 论
本研究检测了HCC患者CD86+M1型TAM的分布情况,结果显示,HCC组织肿瘤间质中存在数量不等的CD86+M1型TAM,CD86+M1型TAM密度与CD8+肿瘤浸润细胞毒性T淋巴细胞密度呈正相关,与HBsAg阳性呈负相关。高密度CD86+M1型TAM是OS与DFS的有利预后因子。 HCC组织表达PD-L1提示肿瘤侵袭性增强、患者预后差。
肿瘤微环境是一个受细胞间通讯调控的动态系统,与肿瘤的发展和转移密切相关。在多种实体瘤中,TAM作为肿瘤微环境中的主要组成细胞,具有高度可塑性,TAM通过整合肿瘤微环境中的信号来调节其活性和表型,在不同的细胞因子或信号刺激下具有不同的表型,主要包括M1型和M2型。M1型TAM在肿瘤生长中发挥抑制作用,而M2型TAM则促进肿瘤生长。Jiang 等[4]观察到M1型TAM可抑制食管鳞状细胞癌细胞的迁移和侵袭。Li等[5]观察到M1型TAM可抑制肿瘤细胞增殖,诱导肿瘤细胞凋亡,发挥抗肿瘤作用。
然而,近年来关于M1型TAM促进肿瘤进展的作用也已得到证实。有研究[6]显示,M1型TAM可使脑胶质瘤细胞的侵袭性增强。Chen等[7]发现,M1型TAM可诱导乳腺癌细胞的上皮-间质转化(epithelial-mesenchymal transition,EMT),并增强细胞的迁移和侵袭能力,靶向M1型TAM可抑制EMT并限制癌细胞的侵袭能力。总之,目前关于M1型TAM对肿瘤的促进或抑制作用仍存在争议,这表明有必要进行更多的研究。本研究发现CD86+的M1型TAM在HCC组织中存在显著浸润,高密度CD86+M1型TAM可抑制肿瘤进展,延长患者的OS和DFS,进一步验证了M1型TAM抑制HCC肿瘤进展的作用。
影响肿瘤生物学行为的因素极其复杂,除了常用临床病理学指标如性别、年龄、肿瘤大小、肝硬化、组织学分级、微血管侵犯等外,肿瘤微环境中肿瘤浸润淋巴细胞(tumor infiltrating lymphocytes,TIL)也越来越引起重视,研究[8]显示TIL与多种肿瘤患者的预后相关。CD8+细胞毒性T淋巴细胞在肿瘤微环境中发挥重要的免疫杀伤功能,其针对肿瘤抗原特异性激活主要取决于抗原提呈细胞(antigen presenting cell,APC)呈递肿瘤抗原的能力。而M1型TAM作为APC为T细胞呈递抗原,通过抗原提呈参与激活适应性免疫反应。本研究显示,M1型TAM密度与CD8+细胞毒性T淋巴细胞密度呈正相关,提示二者可能在肿瘤微环境中发挥重要的免疫协同作用,可能通过TIL影响患者预后。另外本研究组之前也报道了HCC组织中CD8+肿瘤浸润细胞毒性T淋巴细胞表达程序性死亡[蛋白]-1(programmed death-1,PD-1)提示肿瘤进展和术后复发概率增加[9],这些均反映了HCC肿瘤免疫微环境的复杂性。
本研究发现,M1型TAM密度与HBsAg阳性呈负相关。由乙型肝炎病毒(hepatitis B virus, HBV)感染引起的HCC是一个复杂的过程。 Zhang等[10]最新的研究显示,HBV相关HCC中肿瘤细胞与TAM之间的相互作用可增强癌细胞干性和TAM的M2型极化,并且M2型TAM有助于癌细胞干性的维持。Fei等[11]研究显示,HBV相关HCC中microRNA-155(miR-155)影响TAM极化,miR-155过表达显著促进TAM向M2型极化,而miR-155沉默则显著促进M1型极化。此外,miR-155通过靶向SHIP1表达和诱导TAM的M2极化来加速HCC细胞的增殖、迁移和侵袭。以上研究表明,HBV相关HCC中肿瘤微环境更易诱导TAM向M2型极化,而M1型TAM数量相对减少,这可以部分地解释M1型TAM密度与HBsAg阳性呈负相关这一结果。
本课题组之前的研究[3]显示,HCC组织中M2型TAM表达PD-L1与患者预后不良相关。在HCC中活化的TAM产生的肿瘤坏死因子(tumor necrosis factor-α,TNF-α)和白细胞介素-10(interleukin-10,IL-10)通过自分泌方式刺激TAM表达PD-L1,PD-L1+TAM能有效地抑制肿瘤特异性T淋巴细胞免疫,促进肿瘤的生长;这种作用可以通过阻断TAM表达的PD-L1来逆转[12]。同时M1型TAM分泌IL-1β可以诱导HCC细胞中PD-L1的表达[13]。本研究结果显示,在M1型TAM高密度组,PD-L1的高表达显著影响HCC患者的OS和DFS,在M1型TAM低密度组,PD-L1的高表达显著影响OS,而对DFS影响不显著。因此,对于高密度M1型TAM的HCC患者,靶向PD-L1治疗可能具有更好的效果。
综上,HCC组织中存在数量不等的M1型TAM,M1型TAM密度与CD8+肿瘤浸润细胞毒性T淋巴细胞密度呈正相关,与HBsAg阳性呈负相关。HCC组织中存在高密度M1型TAM是有利的预后因素;表达PD-L1可能促进肿瘤细胞的免疫逃逸,导致侵袭性增强、预后变差。本研究结果为HCC的靶向PD-L1免疫治疗以及患者预后评估提供了更多的依据。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
肖锋,许桐林:完成实验,撰写文章;朱琳、肖静文、吴天祺:参与实验;顾春燕:实验设计和监督,指导文章修改,经费支持。
[参考文献]
[1] BOUTILIER A J, ELSAWA S F. Macrophage polarization states in the tumor microenvironment[J]. Int J Mol Sci, 2021, 22(13): 6995.
[2] CHENG K, CAI N, ZHU J H, et al. Tumor-associated macrophages in liver cancer: from mechanisms to therapy[J]. Cancer Commun, 2022, 42(11): 1112-1140.
[3] 肖 锋, 肖静文, 张海燕, 等. 肝细胞癌组织中M2型肿瘤相关巨噬细胞浸润的临床意义[J]. 中华消化杂志, 2023, 43(5): 327-332.
XIAO F, XIAO J W, ZHANG H Y, et al. Clinical significance of infiltration of M2 tumor-associated macrophage in hepatocellular carcinoma[J]. Chin J Dig, 2023, 43(5): 327-332.
[4] JIANG C H, LIANG W H, LI F P, et al. Distribution and prognostic impact of M1 macrophage on esophageal squamous cell carcinoma[J]. Carcinogenesis, 2021, 42(4): 537-545.
[5] LI J, LI N, WANG J. M1 macrophage-derived exosome encapsulated cisplatin can enhance its anti-lung cancer effect[J]. Minerva Med, 2023, 114(5): 634-641.
[6] REN J B, XU B J, REN J H, et al. The importance of M1-and M2-polarized macrophages in glioma and as potential treatment targets[J]. Brain Sci, 2023, 13(9): 1269.
[7] CHEN Z, WU J, WANG L, et al. Tumor-associated macrophages of the M1/M2 phenotype are involved in the regulation of malignant biological behavior of breast cancer cells through the EMT pathway[J]. Med Oncol, 2022, 39(5): 83.
[8] GARRIDO-MARTIN E M, MELLOWS T W P, CLARKE J, et al. M1hot tumor-associated macrophages boost tissue resident memory T cells infiltration and survival in human lung cancer[J]. J Immunother Cancer, 2020, 8(2): e000778.
[9] 肖 锋, 肖静文, 邵建国, 等. PD-1在肝细胞癌肿瘤浸润淋巴细胞中的表达及与预后的相关性[J]. 中国癌症杂志, 2021, 31(5): 419-427.
XIAO F, XIAO J W, SHAO J G, et al. The expression of programmed death 1 in tumor-infiltrating lymphocytes of hepatocellular carcinoma and its correlation with prognosis[J]. China Oncol, 2021, 31(5): 419-427.
[10] ZHANG Q Y, TSUI Y M, ZHANG V X, et al. Reciprocal interactions between malignant cells and macrophages enhance cancer stemness and M2 polarization in HBV-associated hepatocellular carcinoma[J]. Theranostics, 2024, 14(2): 892-910.
[11] FEI Y M, WANG Z W, HUANG M M, et al. MiR-155 regulates M2 polarization of hepatitis B virus-infected tumour associated macrophages which in turn regulates the malignant progression of hepatocellular carcinoma[J]. J Viral Hepat, 2023, 30(5): 417-426.
[12] KUANG D M, ZHAO Q Y, PENG C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1[J]. J Exp Med, 2009, 206(6): 1327-1337.
[13] ZONG Z Y, ZOU J H, MAO R D, et al. M1 macrophages induce PD-L1 expression in hepatocellular carcinoma cells through IL-1β signaling[J]. Front Immunol, 2019, 10: 1643.

