CGP综述|原发性肝癌的流行病学及其危险因素研究进展
时间:2023-12-06 14:34:01 热度:37.1℃ 作者:网络
原发性肝癌(PLC)居世界常见恶性肿瘤的第六位,居恶性肿瘤死亡常见原因的第三位。PLC主要包括肝细胞癌(占75%~85%)和肝内胆管癌(占10%~15%)。2020年全球PLC新发病例约有905677例,死亡病例数高达830180例。世界上PLC发病率最高的地区是亚洲和非洲,其中我国的PLC患者数量约占全球PLC患者的一半。目前,PLC是我国第四位常见恶性肿瘤及第二位恶性肿瘤致死病因,严重威胁人们的生命健康。充分了解PLC的流行病学特点及危险因素,对PLC的防治工作具有重要的意义。本文综述了我国PLC的流行病学特点以及危险因素,以期为我国PLC的防治工作提供参考和借鉴。
01 PLC流行病学
1.1 PLC发病率和死亡率
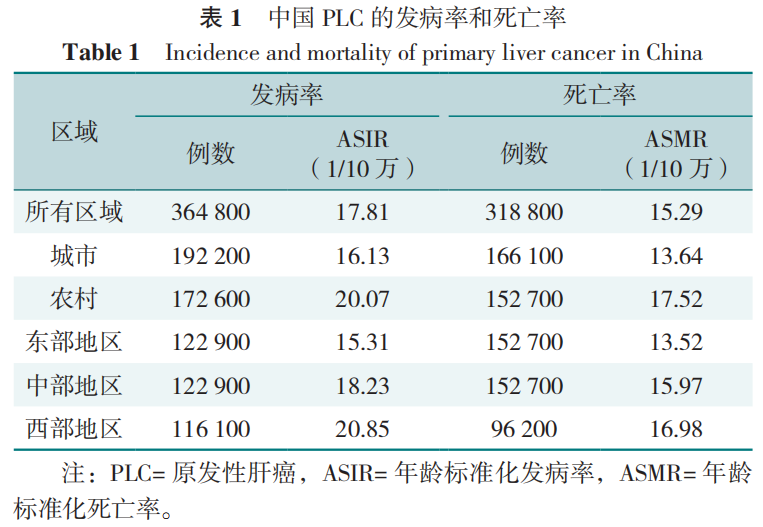
我国人群PLC的年龄标准化发病率(ASIR)和年龄标准化死亡率(ASMR)分别为17.81/10万和15.29/10万;每年PLC总发病例数和死亡例数约占全球一半,并具有显著的城乡和地区差别。其中,农村地区人口ASIR和ASMR均高于城市人口,尤其是65岁以下的人口中城乡差异更显著。在地域分布上,西部欠发达地区的ASIR和ASMR最高,其次是中部、东部地区,见表1。同时,研究也显示,我国2019年PLC的ASIR比1990年下降了58.5%,这可能与乙型肝炎病毒(HBV)和丙型肝炎病毒(HCV)感染患病率和黄曲霉毒素暴露量的下降有关。
1.2 PLC人口学特征
PLC的发病率与年龄密切相关。我国PLC的发病率随年龄增长而逐渐增加,<30岁年龄组的发病率较低,≥30岁年龄组的发病率开始快速升高,80~84岁年龄组的发病率达到高峰。此外,我国PLC发病的年龄呈逐年增长趋势,农村和城市地区男性平均发病年龄由2000年的56.53岁和59.67岁增长至2014年的61.20岁和62.66岁,农村和城市女性则由60.60岁和65.50岁延迟到66.07岁和69.87岁。
在全球大多数地区,男性PLC发病率和死亡率均比女性高2~3倍。在我国,男性PLC发病率和死亡率明显高于女性。这可能与男性和女性的危险因素暴露率不同有关。研究发现,吸烟和饮酒的男性病毒性肝炎患病率高于女性。另有研究显示,雌激素/雄激素水平与HBV转录和复制的多少有关,这可能与男性HBV感染患者中炎症导致PLC的发病率高于女性有关。
1.3 PLC人群归因分值(PAF)
PAF被定义为目标人群中可归因于风险因素的癌症负担量。在全球PLC的发病例数中,归因于HBV感染、HCV感染和饮酒的占比分别为33%、21%和30%。我国72.4%的PLC相关死因归于包括HBV感染、HCV感染、吸烟、饮酒、糖尿病(DM)、肥胖等在内的危险因素。HBV感染在我国男性和女性的PLC负担中占比最大;男性吸烟、饮酒和DM的PAF明显高于女性;然而,与男性肥胖PAF相比,女性肥胖PAF更高,见表2。

此外,不同年龄段的PAF也存在差异。在男性中,HBV引起PLC的PAF在所有年龄段中最大,吸烟和饮酒导致PLC的PAF随年龄增长呈下降趋势。研究发现在女性各年龄组中,HBV引起PLC的PAF也是最大的。男性与女性HCV引起PLC的PAF均随着年龄的增长而升高。在≥60岁的老年人群中,DM、饮酒和吸烟的PAF高于<60岁人群。因此,积极推进HBV疫苗接种、扩大抗病毒治疗、坚持健康的生活方式等是我国PLC一级预防的主要措施。
02 PLC危险因素
PLC常见的危险因素包括慢性HBV、HCV感染、酒精性肝病、代谢性疾病[如非酒精性脂肪性肝病(NAFLD)、DM等]。尽管抗病毒药物可在一定程度上控制乃至根治慢性HBV和HCV感染,但慢性病毒感染仍然是我国PLC的主要病因。此外,随着肥胖、DM人群的增加,代谢综合征(MetS)、NAFLD的流行更加普遍,将进一步导致PLC发病率的上升。
2.1 HBV感染
HBV感染是我国PLC发病的重要危险因素。根据2019年全球疾病负担数据显示,2019年我国HBV感染者约23355000例,HBV相关肝癌新增病例约140000例。HBV感染患者发生PLC的终生风险为10%~25%。HBV还与其他危险因素协同促进PLC的发生。一项荟萃分析显示,男性、饮酒、PLC家族史、DM、未抗病毒治疗以及HBV DNA高复制状态是乙型肝炎肝硬化发生PLC的主要危险因素。因此,在预防和降低PLC的措施方面,HBV疫苗接种是关键。抗病毒治疗也是降低PLC发生风险的有效措施。
2.2 HCV感染
2019年我国约有625000例HCV感染者,HCV相关PLC新增病例约34000例。HCV感染患者一旦进展到肝硬化阶段,PLC的发病率增加为2%~4%。通过抗病毒治疗实现的持续病毒学应答可显著降低HCV相关PLC的发生风险。目前针对HCV相关PLC患者的监测指南中,我国《原发性肝癌的分层筛查与监测指南(2020版)》建议前期已被纳入PLC监测的患者,目前并无停止监测的相关指南,应按原计划继续进行PLC监测。
2.3 吸烟
香烟中含有尼古丁等4000余种有害物质,这些烟草的代谢产物可与DNA结合引发基因突变,增加恶性肿瘤的发生风险。我国一项纳入50万人的前瞻性研究显示,当前吸烟者患PLC的风险比从不吸烟者高28%。研究也显示,戒烟年限与PLC风险呈负相关,戒烟30年的人患PLC的风险与从不吸烟的人相似。
2.4 饮酒
过度饮酒是公认的发生PLC的危险因素。一项荟萃分析表明,酒精相关性肝硬化患者在1年、5年和10年随访时的PLC累积发病率分别为1%、3%和9%。大量饮酒(≥3杯/d)使普通人群的PLC发生风险增加16%。饮酒量越大、饮酒年限越长,PLC的患病风险越高。同时,该研究也显示,控制饮酒和减少饮酒年限有助于预防PLC的发生,特别是>30岁的人群和癌症高发人群应减少酒精的摄入。此外,酒精还和其他危险因素共同促进PLC的发生、发展。一项前瞻性研究显示,饮酒与肥胖可增加PLC的发生风险(HR=3.82)。在酒精相关肝硬化患者中,合并DM比不合并DM患者患PLC的风险高50%。因此,应加强对大量饮酒者的DM筛查,以识别PLC高危患者。
2.5 代谢相关危险因素
2.5.1 DM:
针对不同人群的研究表明,DM与PLC发生风险增加2~3倍相关,男性的相对风险明显高于女性。一项前瞻性研究荟萃分析显示,较长的DM病程与PLC风险的增加有关。此外,DM与NAFLD关系密切。研究显示,DM是NAFLD患者发展为PLC的重要代谢性危险因素。在NAFLD相关肝硬化患者中,DM患者的PLC发生风险较非DM患者升高4.2倍。一项针对85 000例NAFLD合并DM患者进行平均10年随访的研究显示,与没有DM的患者相比,合并DM的患者患PLC的风险高24%(HR=1.24),血糖控制良好的患者与血糖控制欠佳的患者相比,PLC风险降低32%(HR=0.68)。
2.5.2 肥胖:
针对一般人群的荟萃分析显示,肥胖使PLC的风险增加约2倍。美国的队列研究显示,腰围大(定义为男性≥110 cm、女性≥90 cm)的个体罹患PLC的风险增加2倍。除此之外,肥胖还可以增加慢性肝病患者PLC的发生。一项针对慢性HBV患者的中国研究报告称,与非中心性肥胖相比,中心性肥胖(定义为腰围/身高>0.5)与PLC风险增加相关(HR=1.63)。此外,肥胖与NAFLD关系密切,共同促进PLC的发生。
2.5.3 MetS:
MetS是PLC的危险因素,包括腹部肥胖、高甘油三酯血脂、低高密度脂蛋白胆固醇血症、高血压以及高血糖。一项中位随访时间为13.02年的前瞻性研究显示,MetS患者发生PLC的风险是非MetS患者的2.91倍。另一项队列研究显示,在校正年龄、性别和其他合并症后,MetS合并NAFLD的患者发生PLC的风险是对照组(非MetS和非NAFLD患者)的15.33倍。
2.5.4 NAFLD:
随着肥胖和MetS的流行,NALFD导致的PLC发病率呈逐年上升趋势。一项研究发现,NAFLD患者的PLC发病率为0.21/1000人年,显著高于非NAFLD患者(0.02/1 000人年)。每增加一个额外的代谢因素(包括DM、肥胖、血脂异常和高血压),NAFLD患者发生PLC的风险也会增加,其中伴有DM的NAFLD患者进展为PLC的风险是非DM患者的2.77倍,提示临床医生注意对伴有代谢因素的NAFLD患者开展PLC筛查工作。
2.6 其他因素
黄曲霉毒素是一类由黄曲霉、寄生曲霉等真菌产生的致癌物质,其中黄曲霉毒素B1(AFB1)的毒性最大,致癌性最强。一方面,AFBl可诱发急性肝坏死、导致肝硬化或PLC;另一方面,AFBl的代谢产物可通过环氧化物代谢物结合DNA和烷基化碱基,诱导细胞周期紊乱和p53的基因突变,增加PLC的发生风险。自1985年以来,允许水稻代替玉米的政策已经使黄曲霉毒素生物标志物降低了97.4%,这使得我国PLC的发病率有所下降。
蓝藻分泌的微囊藻毒素(MC)是一类天然的具有肝毒性的代谢产物,普遍存在于淡水湖和饮用水中。MC主要通过抑制蛋白磷酸酶1和2A导致中间丝和微丝的过度磷酸化和肝细胞骨架的损伤,从而诱导PLC的发生。
03 小结和展望
目前,HBV和HCV感染仍然是PLC最重要的危险因素。伴随HBV疫苗的接种、对HBV和HCV感染患者有效的抗病毒治疗以及对病毒性肝炎高危人群的筛查,病毒性肝炎的患病率将会有所下降。肥胖、DM、NAFLD以及过量饮酒等因素是PLC发生的重要危险因素。普及并推广健康的生活方式,加大对NAFLD等代谢性疾病的筛查、预防和治疗,完善PLC监测手段并不断精进治疗策略,将有力地提高我国PLC的防治水平,从而在未来全面降低我国PLC的社会、经济和医疗负担。
然而,目前仍有亟需研究和解决的问题:(1)目前缺乏特异性识别早期PLC的生物标志物及监测手段;(2)缺乏PLC高危人群的简便分层识别工具,如可以研发自我监测的APP;(3)缺乏治疗NAFLD的有效方法。希望在不久的将来能解决上述问题,减轻全球PLC负担。
参考文献
[1] SUNG H,FERLAY J,SIEGEL R L,et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249.
[2] PETRICK J L,FLORIO A A,ZNAOR A,et al. International trends in hepatocellular carcinoma incidence,1978-2012[J]. Int J Cancer,2020,147(2):317-330.
[3] LLOVET J M,KELLEY R K,VILLANUEVA A,et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers,2021,7(1):6.
[4] CAO W,CHEN H D,YU Y W,et al. Changing profiles of cancer burden worldwide and in China:a secondary analysis of the global cancer statistics 2020[J]. Chin Med J,2021,134(7):783-791.
[5] ZHENG R S,QU C F,ZHANG S W,et al. Liver cancer incidence and mortality in China:temporal trends and projections to 2030[J]. Chin J Cancer Res,2018,30(6):571-579.
[6] YU S X,WANG H W,HU T Y,et al. Disease burden of liver cancer attributable to specific etiologies in China from 1990 to 2019:an age-period-cohort analysis[J]. Sci Prog,2021,104(2):368504211018081.
[7] ZOU Z Y,ZHANG Z F,LU C,et al. Comparison of time trends in the incidence of primary liver cancer between China and the United States:an age-period-cohort analysis of the Global Burden of Disease 2019[J]. Chin Med J,2022,135(17):2035-2042.
[8] 安澜,曾红梅,郑荣寿,等. 2015年中国肝癌流行情况分析[J]. 中华肿瘤杂志,2019,41(10):721-727.
[9] 曾红梅,曹毛毛,郑荣寿,等. 2000—2014年中国肿瘤登记地区肝癌发病年龄变化趋势分析[J]. 中华预防医学杂志,2018,52(6):573-578.
[10] CAO M M,DING C,XIA C F,et al. Attributable deaths of liver cancer in China[J]. Chin J Cancer Res,2021,33(4):480-489.
[11] MCGLYNN K A,PETRICK J L,EL-SERAG H B. Epidemiology of hepatocellular carcinoma[J]. Hepatology,2021,73(Suppl 1):4-13.
[12] WU E M,WONG L L,HERNANDEZ B Y,et al. Gender differences in hepatocellular cancer:disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation[J]. Hepatoma Res,2018,4:66.
[13] MAUCORT-BOULCH D,MARTEL C D,FRANCESCHI S,et al. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide[J]. Int J Cancer,2018,142(12):2471-2477.
[14] 陈万青,崔富强,樊春笋,等. 中国肝癌一级预防专家共识(2018)[J]. 临床肝胆病杂志,2018,34(10):2090-2097.
[15] GAO Q,ZHU H W,DONG L Q,et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma[J]. Cell,2019,179(5):1240.
[16] JIANG Y,HAN Q J,ZHAO H J,et al. The mechanisms of HBV-induced hepatocellular carcinoma[J]. J Hepatocell Carcinoma,2021,8:435-450.
[17] CHEN Y Y,WANG W H,CHE L,et al. BNIP3L-dependent mitophagy promotes HBx-induced cancer stemness of hepatocellular carcinoma cells via glycolysis metabolism reprogramming[J]. Cancers,2020,12(3):655.
[18] YUE T T,ZHANG Q Q,CAI T,et al. Trends in the disease burden of HBV and HCV infection in China from 1990-2019[J]. Int J Infect Dis,2022,122:476-485.
[19] MCGLYNN K A,PETRICK J L,LONDON W T. Global epidemiology of hepatocellular carcinoma:an emphasis on demographic and regional variability[J]. Clin Liver Dis,2015,19(2):223-238.
[20] 陈曦阳光,吴君. 乙型肝炎肝硬化并发原发性肝癌相关危险因素Meta分析[J]. 肝脏,2019,24(4):398-404.
[21] HUANG J,LIU Y Q,LIU Y S. Antiviral therapy in hepatitis B virus-infected with immune-tolerant:a meta-analysis[J]. Gastroenterol Hepatol,2023,46(4):309-318.
[22] D'SOUZA S,LAU K C,COFFIN C S,et al. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma[J]. World J Gastroenterol,2020,26(38):5759-5783.
[23] THYLUR R P,ROY S K,SHRIVASTAVA A,et al. Assessment of risk factors,and racial and ethnic differences in hepatocellular carcinoma[J]. JGH Open,2020,4(3):351-359.
[24] PAWLOTSKY J M,NEGRO F,AGHEMO A,et al. EASL recommendations on treatment of hepatitis C:Final update of the series[J]. J Hepatol,2020,73(5):1170-1218.
[25] MA L T,LIU J L,WANG W,et al. Direct-acting antivirals and interferon-based therapy on hepatocellular carcinoma risk in chronic hepatitis-C patients[J]. Future Oncol,2020,16(11):675-686.
[26] 丁惠国,屠红,曲春枫,等. 原发性肝癌的分层筛查与监测指南(2020版)[J]. 临床肝胆病杂志,2021,37(2):286-295.
[27] PANG Q,QU K,ZHANG J Y,et al. Cigarette smoking increases the risk of mortality from liver cancer:a clinical-based cohort and meta-analysis[J]. J Gastroenterol Hepatol,2015,30(10):1450-1460.
[28] WEN Q R,CHAN K H,SHI K X,et al. Tobacco smoking and solid fuels for cooking and risk of liver cancer:a prospective cohort study of 0.5 million Chinese adults[J]. Int J Cancer,2022,151(2):181-190.
[29] PETRICK J L,CAMPBELL P T,KOSHIOL J,et al. Tobacco,alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma:the Liver Cancer Pooling Project[J]. Br J Cancer,2018,118(7):1005-1012.
[30] KONYN P,AHMED A,KIM D. Current epidemiology in hepatocellular carcinoma[J]. Expert Rev Gastroenterol Hepatol,2021,15(11):1295-1307.
[31] TANIAI M. Alcohol and hepatocarcinogenesis[J]. Clin Mol Hepatol,2020,26(4):736-741.
[32] HUANG D Q,TAN D J H,NG C H,et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis:systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol,2023,21(5):1169-1177.
[33] TURATI F,GALEONE C,ROTA M,et al. Alcohol and liver cancer:a systematic review and meta-analysis of prospective studies[J]. Ann Oncol,2014,25(8):1526-1535.
[34] HE F D,SHA Y T,WANG B H. Relationship between alcohol consumption and the risks of liver cancer,esophageal cancer,and gastric cancer in China:Meta-analysis based on case-control studies[J]. Medicine,2021,100(33):e26982.
[35] LOOMBA R,YANG H I,SU J,et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma:a prospective cohort study[J]. Am J Epidemiol,2013,177(4):333-342.
[36] SHI T T,KOBARA H,OURA K,et al. Mechanisms underlying hepatocellular carcinoma progression in patients with type 2 diabetes[J]. J Hepatocell Carcinoma,2021,8:45-55.
[37] OHKUMA T,PETERS S A E,WOODWARD M. Sex differences in the association between diabetes and cancer:a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events[J]. Diabetologia,2018,61(10):2140-2154.
[38] SIMON T G,KING L Y,CHONG D Q,et al. Diabetes,metabolic comorbidities,and risk of hepatocellular carcinoma:results from two prospective cohort studies[J]. Hepatology,2018,67(5):1797-1806.
[39] KANWAL F,KRAMER J R,LI L,et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease[J]. Hepatology,2020,71(3):808-819.
[40] YANG J D,AHMED F,MARA K C,et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease[J]. Hepatology,2020,71(3):907-916.
[41] KRAMER J R,NATARAJAN Y,DAI J L,et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease[J]. Hepatology,2022,75(6):1420-1428.
[42] BESSONE F,RAZORI M V,ROMA M G. Molecular pathways of nonalcoholic fatty liver disease development and progression[J]. Cell Mol Life Sci,2019,76(1):99-128.
[43] CHONG L W,TSAI C L,YANG K C,et al. Targeting protein palmitoylation decreases palmitate-induced sphere formation of human liver cancer cells[J]. Mol Med Rep,2020,22(2):939-947.
[44] BROADFIELD L A,DUARTE J A G,SCHMIEDER R,et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis[J]. Cancer Res,2021,81(8):1988-2001.
[45] RAJESH Y,SARKAR D. Association of adipose tissue and adipokines with development of obesity-induced liver cancer[J]. Int J Mol Sci,2021,22(4):2163.
[46] HUANG D Q,EL-SERAG H B,LOOMBA R. Global epidemiology of NAFLD-related HCC:trends,predictions,risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol,2021,18(4):223-238.
[47] GUPTA A,DAS A,MAJUMDER K,et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality:a systematic review and meta-analysis[J]. Am J Clin Oncol,2018,41(9):874-881.
[48] FLORIO A A,CAMPBELL P T,ZHANG X H,et al. Abdominal and gluteofemoral size and risk of liver cancer:the liver cancer pooling project[J]. Int J Cancer,2020,147(3):675-685.
[49] FAN R,NIU J Q,MA H,et al. Association of central obesity with hepatocellular carcinoma in patients with chronic hepatitis B receiving antiviral therapy[J]. Aliment Pharmacol Ther,2021,54(3):329-338.
[50] CHEN J,SONG S,LI X S,et al. Association of metabolic traits with occurrence of nonalcoholic fatty liver disease-related hepatocellular carcinoma:a systematic review and meta-analysis of longitudinal cohort studies[J]. Saudi J Gastroenterol,2022,28(2):92-100.
[51] SONG M M,LIU T,LIU H,et al. Association between metabolic syndrome,C-reactive protein,and the risk of primary liver cancer:a large prospective study[J]. BMC Cancer,2022,22(1):853.
[52] CHEN Y G,YANG C W,CHUNG C H,et al. Correction to:The association between metabolic risk factors,nonalcoholic fatty liver disease,and the incidence of liver cancer:a nationwide population-based cohort study[J]. Hepatol Int,2022,16(2):488.
[53] FUCHS A,SAMOVSKI D,SMITH G I,et al. Associations among adipose tissue immunology,inflammation,exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease[J]. Gastroenterology,2021,161(3):968-981.e12.
[54] PARTHASARATHY G,REVELO X,MALHI H. Pathogenesis of nonalcoholic steatohepatitis:an overview[J]. Hepatol Commun,2020,4(4):478-492.
[55] ESLAM M,VALENTI L,ROMEO S. Genetics and epigenetics of NAFLD and NASH:clinical impact[J]. J Hepatol,2018,68(2):268-279.
[56] PAIK J M,GOLABI P,YOUNOSSI Y,et al. The growing burden of disability related to nonalcoholic fatty liver disease:data from the global burden of disease 2007-2017[J]. Hepatol Commun,2020,4(12):1769-1780.
[57] KANWAL F,KRAMER J R,MAPAKSHI S,et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease[J]. Gastroenterology,2018,155(6):1828-1837.e2.
[58] KANWAL F,KRAMER J R,LI L,et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease[J]. Hepatology,2020,71(3):808-819.
[59] TANG A,HALLOUCH O,CHERNYAK V,et al. Epidemiology of hepatocellular carcinoma:target population for surveillance and diagnosis[J]. Abdom Radiol,2018,43(1):13-25.
[60] AI Y Q,HUANG K L,ZHANG B Y,et al. Aflatoxin B1-induced epigenetic alterations:an overview[J]. Food Chem Toxicol,2017,109(Pt 1):683-689.
[61] QI L N,BAI T,CHEN Z S,et al. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi,China:role of chronic hepatitis B virus infection and aflatoxin B1 exposure[J]. Liver Int,2015,35(3):999-1009.
[62] CHEN J G,EGNER P A,NG D,et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China[J]. Cancer Prev Res,2013,6(10):1038-1045.
[63] FUJIKI H,SUGANUMA M. Tumor promoters—microcystin-LR,nodularin and TNF-α and human cancer development[J]. Anticancer Agents Med Chem,2011,11(1):4-18.
[64] HERNANDEZ B Y,ZHU X M,RISCH H A,et al. Oral cyanobacteria and hepatocellular carcinoma[J]. Cancer Epidemiol Biomarkers Prev,2022,31(1):221-229.


