【论著】| TMCO1对宫颈癌细胞增殖和迁移能力的影响
时间:2024-07-21 06:00:37 热度:37.1℃ 作者:网络
[摘要] 背景与目的:跨膜和卷曲螺旋结构域1(transmembrane and coiled-coil domains 1,TMCO1)是新近发现的一种内质网钙离子通道蛋白,已被发现与多种肿瘤的进展相关,但其在宫颈癌中的作用尚未明确。本研究旨在探讨TMCO1对宫颈癌HeLa细胞增殖和迁移能力的影响。方法:通过质粒转染宫颈癌HeLa细胞,获得稳定过表达TMCO1的细胞株和稳定敲减TMCO1的细胞株。采用细胞计数试剂盒-8(cell counting kit-8,CCK-8)实验、克隆形成实验和EdU标记实验检测细胞增殖能力,采用transwell实验检测细胞迁移能力,并将稳定过表达TMCO1细胞与对照细胞进行蛋白质组学分析。结果:CCK-8实验和克隆形成实验显示,宫颈癌HeLa细胞过表达TMCO1后,增殖能力显著增加(P<0.05);EdU标记实验显示,宫颈癌HeLa细胞过表达TMCO1后,进行活跃DNA合成的细胞数量明显增多(P<0.01)。宫颈癌HeLa细胞敲减TMCO1后,细胞周期抑制蛋白p27表达增加,组蛋白H3的磷酸化减弱;克隆形成实验显示,敲减TMCO1显著抑制宫颈癌HeLa细胞的增殖(P<0.001);EdU标记实验显示,宫颈癌HeLa细胞敲减TMCO1后,进行活跃DNA合成的细胞数量明显减少(P<0.05)。Transwell实验显示,宫颈癌HeLa细胞过表达TMCO1后,迁移能力显著增加(P<0.001);敲减TMCO1显著抑制宫颈癌HeLa细胞的迁移(P<0.001)。稳定过表达TMCO1细胞中与细胞外基质-黏附及磷脂酰肌醇3-激酶(phosphoinositide3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)信号转导通路显著激活,而核糖体相关等通路受到抑制。结论:TMCO1过表达显著促进宫颈癌HeLa细胞的增殖和迁移,敲减TMCO1显著抑制宫颈癌HeLa细胞的增殖和迁移,TMCO1可能通过调节细胞的黏附及信号转导影响HeLa细胞的增殖和迁移。
[关键词] 宫颈癌;TMCO1;增殖;迁移
[Abstract] Background and purpose: Transmembrane and coiled-coil domains 1 (TMCO1) is a recently discovered endoplasmic reticulum calcium channel protein that has been found to be associated with the progression of various tumors, however, its role in cervical cancer has not yet been clarified. This study aimed to investigate the effects of TMCO1 on the proliferation and migration of cervical cancer HeLa cells. Methods: By transfecting cervical cancer HeLa cells with plasmids, cells with stable overexpression of TMCO1 and cells with stable knockdown of TMCO1 were obtained. Cell counting kit-8 (CCK-8) assay, clone formation assay and EdU labeling assay were used to detect cell proliferation ability, transwell assay was used to detect cell migration ability, and proteomic analysis was performed on the cells that stably overexpressed TMCO1 and control cells. Results: TThe CCK-8 experiment and clone formation experiment showed that overexpression of TMCO1 in cervical cancer HeLa cells significantly increased their proliferation ability (P<0.05). EdU labeling experiments showed that overexpression of TMCO1 in cervical cancer HeLa cells significantly increased the number of cells undergoing active DNA synthesis (P<0.01). After knocking down TMCO1 in cervical cancer HeLa cells, the expression of cell cycle inhibitory protein p27 increased, and the phosphorylation of histone H3 decreased. Clonogenesis experiments showed that knocking down TMCO1 significantly inhibited the proliferation of cervical cancer HeLa cells (P<0.001). EdU labeling experiments showed that after knocking down TMCO1 in cervical cancer HeLa cells, the number of cells undergoing active DNA synthesis was significantly reduced (P<0.05). Transwell experiment showed that overexpression of TMCO1 in cervical cancer HeLa cells significantly increased their migration ability (P<0.001), while knocking down TMCO1 significantly inhibited the migration of cervical cancer HeLa cells (P<0.001). The pathways related to extracellular matrix adhesion and PI3K-AKT signaling were significantly upregulated in the cells with stable overexpression of TMCO1, while ribosome related pathways were downregulated in proteomic analysis. Conclusion: Overexpression of TMCO1 significantly promotes the proliferation and migration of cervical cancer cells, while knockdown of TMCO1 significantly inhibits the proliferation and migration of cervical cancer HeLa cells. TMCO1 may affect the proliferation and migration of HeLa cells by regulating cell adhesion and signal transduction.
[Key Words] Cervical cancer; TMCO1; Proliferation; Migration
宫颈癌是妇科常见恶性肿瘤之一,主要治疗手段包括手术、化疗、放疗等,肿瘤局部进展和远处转移是宫颈癌患者死亡的主要原因。美国癌症协会数据显示,近十年宫颈癌患者的5年生存率没有明显提高[1-2]。中国已采取一级预防和二级预防措施,宫颈癌2020年新增病例数从2015年的11.1万降至11万;但同年死于宫颈癌的人数却从2015年的3.4万上升至5.9万,已经罹患宫颈癌,尤其晚期、转移和复发的宫颈癌患者,预后并不乐观[3]。因此,有必要开展有针对性的基础研究,以寻求新的应对策略。
钙离子是重要的第二信使,参与调控细胞多种生理活动,如增殖、迁移、死亡等。内质网是细胞内主要的钙库,通过与其他细胞器和质膜协调,在维持钙稳态方面发挥着重要作用[4]。Li等[5]在宫颈癌HeLa细胞的研究中发现,内质网钙稳态功能被破坏后能导致内质网应激相关细胞死亡。跨膜和卷曲螺旋结构域1(transmembrane and coiled-coil domains 1,TMCO1)是新近发现的一种内质网钙离子通道蛋白,又被称为内质网钙过载激活的钙离子通道,当内质网出现钙过载时,TMCO1可以感知内质网中过高的钙浓度并将内质网中过多的钙离子排出,从而消除内质网的钙过载,维持细胞钙稳态[6],其表达异常会引起内质网的钙稳态失衡,从而影响钙信号转导及相应的细胞功能[7]。Sun等[8]研究发现,TMCO1对卵泡发育和女性生育能力至关重要,敲减TMCO1会导致卵泡发育受损。Li等[9]研究发现,骨质疏松症患者的骨中TMCO1水平显著降低,TMCO1在成骨过程中发挥关键作用。TMCO1缺陷会导致颅面畸形、骨骼异常、整体发育迟缓等多种疾病[10]。
TMCO1在宫颈癌中的作用目前未见报道。本研究拟通过TCGA公共数据库数据,分析TMCO1在宫颈癌中的表达及其与宫颈癌患者预后之间的关系,并进行细胞功能学实验,探索TMCO1对宫颈癌HeLa细胞增殖和迁移能力的影响,为宫颈癌寻找新的治疗靶点提供依据。
1 材料和方法
1.1 公共数据获取和分析
我们首先从UCSC Xena数据库(https://xenabrowser.net/)下载了经统一标准化的泛癌数据集:TCGA TARGET GTEx (PANCAN, N=19131, G=60499),从中提取了ENSG00000143183(TMCO1)基因在各个样本中的表达数据,并筛选了来源为宫颈癌的样本,进一步对每一个表达值进行了log2(x+0.001)变换。我们对304个肿瘤样本和13个癌旁样本进行TMCO1表达水平的差异检测,并使用秩和检验分析差异的显著性。
我们又从TCGA公共数据库获取宫颈癌患者的FPKM(Fragments Per Kilobase Million)转录组表达数据以及随访资料。从GENCODE (https://www.gencodegenes.org/human/)中下载了v22版本的gff3(http://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_22/gencode.v22.annotation.gff3.gz)文件和v33版本的gff3(http://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_33/gencode. v33.annotation.gff3.gz)文件,从中提取了GeneSymbol和ENSG_ID的mapping信息,将ENSG_ID映射到GeneSymbol上,当存在多个匹配时,取中位数,最终获得了转换后的FPKM表达谱。我们使用R软件包maxstat(maximally selected rank statistics with several P-value approximations version: 0.7-25)计算了TMCO1的最佳截断值,设置最小分组样本数大于25%,最大样本数分组小于75%,最终获得最佳截断值为20.00434,基于此将患者分成高低两组。
1.2 试剂
细胞计数试剂盒-8(cell counting kit-8,CCK-8)购自英国Abcam公司,DMEM培养基购自上海源培生物科技股份有限公司,磷酸酶抑制剂、蛋白酶抑制剂混合液购自美国Selleck公司,嘌呤霉素购自美国Invitrogen公司,PVDF膜购自美国BIORAD公司,甲醇购自美国Sensi Chemical公司,青霉素/链霉素、胰蛋白酶、低熔点琼脂糖凝胶购自美国Gibco公司,结晶紫染色液、BeyoClickTM EdU-488细胞增殖检测试剂盒购自上海碧云天生物技术股份有限公司,抗p-H3抗体购自美国Cell signaling公司,聚凝胺、抗Flag抗体、抗Vinculin抗体购自美国Sigma公司,抗P27抗体购自美国BD公司,抗TMCO1抗体购自美国Proteintech公司。
1.3 质粒
pLex-Flag-TMCO1、PLKO.1-shTMCO1-1、 PLKO.1-shTMCO1-2均为厦门大学附属中山医院中心实验室构建。shTMCO1-1序列为CCGGCCC TAATGGGAATGTTCAATTCTCGAGAATTGAA CATTCCCATTAGGGTTTTTG;shTMCO1-2序列为CCGGCTTGAACTCAAGAACTCTTTACTCGA GTAAAGAGTTCTTGAGTTCAAGTTTTTG。
1.4 蛋白质印迹法(Western blot)检测
细胞培养后弃去培养皿中的培养基,用磷酸盐缓冲生理盐水(phosphate-buffered saline,PBS)洗涤1次,吸干PBS。使用预冷的EBC缓冲液(50 mmol/L Tris-HCl pH 8.0, 120 mmol/L NaCl,0.5% NP-40)含1%蛋白酶抑制剂和1%磷酸酶抑制剂,将细胞从培养皿中刮下,转移到离心管,置于冰上。细胞样品在4 ℃冷库旋转裂解20 min后,12 000 r/min 4 ℃离心1 min。用细胞超声裂解仪破碎细胞。以12 000 r/min 4 ℃离心10 min,将上清液转移至另一个空的离心管中。使用Bradford法测量蛋白浓度,然后再加入1/2上清液体积的3×loading buffer并混匀。100 ℃煮样5 min后,用Vortex(涡旋混合仪)混匀1~2 s后以12 000 r/min速率离心1 min后进行蛋白凝胶电泳。
1.5 CCK-8和克隆形成实验
将细胞消化,离心,重悬,按照一定比例稀释细胞,计数。
1.5.1 CCK-8实验
向96孔板中加入100 μL包含1 000个细胞的细胞悬液,设置3个复孔,96孔板周围加入PBS,在固定时间点移除培养基,加入含有10% CCK-8试剂的100 μL培养基,使用不含细胞的孔作为对照。培养箱培养2 h后,使用分光光度仪测量吸光度(D)值,连续测量5 d,使用软件GraphPad Prism 8进行分析作图,统计细胞生长增殖差异并绘制生长曲线。
1.5.2 克隆形成实验
6孔板中加入2 mL培养基1 000个细胞,生长10 d,弃培养基,PBS洗1遍,弃PBS,加入1 mL结晶紫染色5 min,之后清洗,晾干并拍照。
1.6 Transwell实验
将细胞消化、离心并用无血清培养基重悬细胞然后计数。将transwell小室置于24孔板,小室下方加入600 μL含有10%血清的培养基,小室中加入200 μL包含30 000个细胞的无血清培养基,培养箱放置24 h。取出transwell小室,弃去培养基,用PBS洗2遍。甲醇固定30 min,弃去甲醇,小室适当晾干。加入结晶紫固定20 min。回收结晶紫,PBS清洗3遍。用棉签轻轻擦去上层未迁移细胞,晾干。用显微镜观察细胞,拍照计数。
1.7 EdU标记实验
将细胞消化、离心,用培养基重悬然后计数。在24孔板中(提前加入盖玻片)培养40 000个细胞,细胞培养过夜并且恢复到正常状态。配制2×EdU 工作液:由于EdU工作液是与培养液等体积加入到孔板中,所以需要配制成2×工作液,加入之后终浓度为10 μmol/L。将37 ℃预热的2×EdU工作液(20 μmol/L),等体积加入24孔板中,使24孔板中的EdU终浓度变为1×。继续温育细胞2 h。EdU标记细胞完成后,去除培养液并加入0.5 mL 固定液(4%的多聚甲醛),室温固定15 min。去除固定液,每孔用0.5 mL洗涤液[含3%的牛血清白蛋白(bovine serum albumin,BSA)的PBS]洗涤细胞3次,每次5 min。去除洗涤液,每孔用500 μL通透液(含0.3% Triton X-100的PBS),室温温育15 min。去除通透液,每孔用0.5 mL洗涤液洗涤细胞2次,每次5 min。配制Click反应液(1 mL体积反应液含有860 μL Click Reaction Buffer,40 μL CuSO4,2 μL Azide 488,100 μL Click Additive Solution)。去除上一步骤中的洗涤液,每孔加入100 μL Click反应液,轻轻摇晃培养板以确保反应混合物可以均匀覆盖样品。室温避光温育30 min。吸除Click反应液,用洗涤液洗涤3次,每次5 min。吸除洗涤液后,每孔加1×Hoechst 33342溶液200 μL,室温避光温育10 min。吸除1×Hoechst 33342溶液,用洗涤液洗涤3次,每次5 min。随后即可进行荧光检测。在488 nm波长激发光条件下检测EdU绿色荧光,在346 nm波长激发光条件下检测Hoechst 33342蓝色荧光,拍照统计。
1.8 蛋白质组学分析
收取细胞进行裂解后,计算各样品的蛋白质浓度后按胰蛋白酶与蛋白质量比1∶50进行过夜酶解,之后进行脱盐纯化。对脱盐后的肽段样品的质谱分析在串联了EASY-nLC 1 000 HPLC系统的Q-Exactive HF质谱仪(美国Thermo Fisher Scientific公司)上进行。使用的色谱柱为75 μm×200 mm的毛细管柱,内部填有3 μm粒径的ReproSil-Pμr C18填料(德国Dr Maisch公司)。样品以90 min的色谱梯度分离,流速为300 nL/min。质谱仪Q Exactive HF的喷雾电压为2 300 V,正离子模式,离子传输管的温度设定为300 ℃。利用Xcalibur软件来进行质谱数据采集,采集模式为DIA。参数设置如下:MS1全扫描分辨率为120 000@m/z 200,AGC为3e6,最大IT时间为100 ms(35~1 650 m/z)。对采集数据使用DIA-NN 1.8.1进行搜库,采用的是无谱图库DIA数据搜索方法,数据库为含2 0607条序列的human Swiss-Prot数据库。DIA-NN搜库产生的结果中,文件“report.unique_genes_matrix. tsv”用于后续分析。蛋白质组学数据采用中位数法归一化,进行log2转化,数据进一步使用R进行生物信息学分析,主要包括使用limma进行差异分析,使用clμsterProfiler进行富集分析,使用ggplot2绘图。
1.9 统计学处理
使用R软件包survival的survfit函数分析宫颈癌患者FPKM高低两组的预后差异,利用对数秩检验(log-rank)方法评估不同组样本之间的预后差异显著性。采用SPSS 22.0软件进行统计学分析。细胞各实验组每种条件均设置3个独立重复,两两数据之间比较采用Student t检验。P <0.05为差异有统计学意义。
2 结 果
2.1 TMCO1在宫颈癌中高表达,且提示预后不良
从箱式图可以看出,TMCO1在肿瘤组织中的表达水平较癌旁组织高,差异有统计学意义(P<0.05,图1A)。
本研究从TCGA公共数据库共获取291位女性宫颈癌患者的TMCO1 mRNA表达水平及总生存数据,有220位在随访终点存活,71位死亡。以TMCO1表达水平20.00434 FPKM作为区分高低表达的临界值,根据生存时间进行绘图(图1B),纵坐标为生存的概率,横坐标为生存的天数,结果显示,TMCO1的高表达与宫颈癌患者的不良预后明显相关(P=0.003 3)。
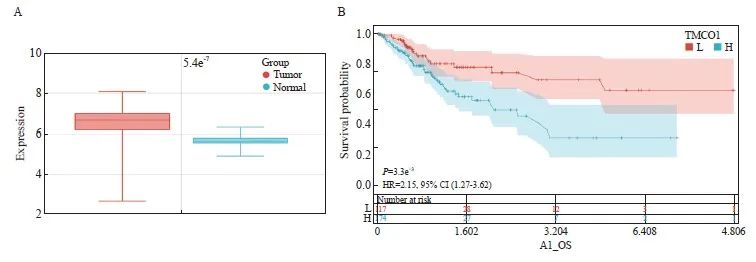
图1 宫颈癌TMCO1 mRNA的表达及其与生存的关系
Fig. 1 Expression of TMCO1 mRNA in cervical cancer and its relationship with survival
A: Differential expression of TMCO1 in cervical cancer and adjacent normal tissues. B: Survival curves of different TMCO1 mRNA expression levels in cervical cancer.
2.2 过表达TMCO1促进HeLa细胞增殖,敲减TMCO1抑制HeLa细胞增殖
通过制备带有N端融合Flag标签的TMCO1慢病毒表达载体,感染宫颈癌HeLa细胞,获得稳定表达TMCO1的细胞,感染空载体(EV)的细胞作为对照,并通过Western blot法确认TMCO1的表达(图2A)。
将上述的稳转Flag-TMCO1细胞及对照细胞(EV)进行CCK-8细胞增殖实验,按照试剂盒说明每天收取细胞进行检测,且与接种细胞当天的数值(第0天)进行比较获得相对值。CCK-8实验结果显示,宫颈癌HeLa细胞过表达TMCO1后,增殖能力显著增加(P<0.05,图2B)。
将上述的稳转Flag-TMCO1细胞及对照细胞(EV)按1 000个细胞/孔接种至6孔板,培养10天后结晶紫染色并统计克隆形成数量。以感染空载体(EV)的细胞形成克隆数为1.0,获得稳转Flag-TMCO1细胞形成克隆数的相对值。克隆形成实验显示,TMCO1过表达显著促进宫颈癌HeLa细胞的增殖(P<0.05,图2C、D)。
将上述的稳转Flag-TMCO1细胞及对照细胞(EV)进行EdU标记,以检测进行活跃DNA合成的细胞数量。按照实验步骤要求,分别计数EdU标记(绿色荧光)的细胞相对比例(除以同一视野中细胞的数量,Hoechst用于标记细胞核)。EdU标记实验显示,宫颈癌HeLa细胞过表达TMCO1后,进行活跃DNA合成的细胞数量明显增多(P<0.01,图2E、F)。
通过设计两条独立的靶向TMCO1基因序列的shRNA,并克隆入pLKO.1慢病毒载体,感染宫颈癌细胞HeLa,经嘌呤霉素筛选获得稳定敲减TMCO1的细胞,并以感染空载体(pLKO.1)病毒的细胞作为对照。收取细胞通过Western blot法检测TMCO1的敲减情况,以及细胞周期抑制蛋白p27、组蛋白H3的磷酸化情况。宫颈癌HeLa细胞敲减TMCO1后,细胞周期抑制蛋白p27表达增加,组蛋白H3的磷酸化减少(图3A)。
将上述的TMCO1稳定敲减细胞及对照细胞按1 000个细胞/孔接种至6孔板,培养10 d后结晶紫染色并统计克隆形成数量。以感染空载体(pLKO.1)的细胞形成克隆数为1.0,获得TMCO1稳定敲减细胞形成克隆数的相对值。克隆形成实验显示,敲减TMCO1显著抑制宫颈癌HeLa细胞的增殖(P<0.001,图3B、C)。
将上述的TMCO1稳定敲减细胞及对照细胞进行EdU标记,以检测进行活跃DNA合成的细胞数量。按照实验步骤要求,分别计数EdU标记(绿色荧光)的细胞相对比例(除以同一视野中细胞的数量,Hoechst用于标记细胞核)。EdU标记实验显示,宫颈癌HeLa细胞敲减TMCO1后,进行活跃DNA合成的细胞数量明显减少(P<0.05,图3D、E)。
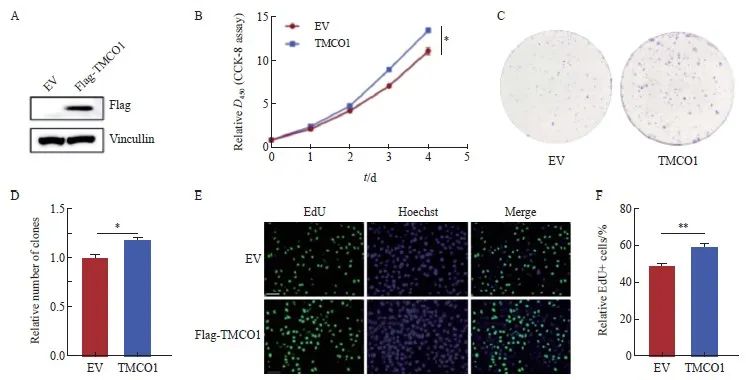
图2 过表达TMCO1促进宫颈癌HeLa细胞的增殖
Fig. 2 Overexpression of TMCO1 promotes the proliferation of cervical cancer HeLa cells
A: A TMCO1 lentiviral expression vector was infected with the cervical cancer cell line HeLa to obtain a cell line stably expressing TMCO1. The cell line infected with the EV was used as a control, and the expression of TMCO1 was confirmed by IB. B: The stably transfected TMCO1 cells and control cells (EV) were subjected to CCK-8 cell proliferation assay. C, D: The stably transfected TMCO1 cells and control cells (EV) were seeded into 6-well plates at 1 000 cells/well, and the number of clones formed was counted after 10 days of culture. E, F: The stably transfected TMCO1 cells and control cells (EV) were labeled with EdU to detect the number of cells undergoing active DNA synthesis. *: P<0.05, **: P<0.01. EV: Empty vector.
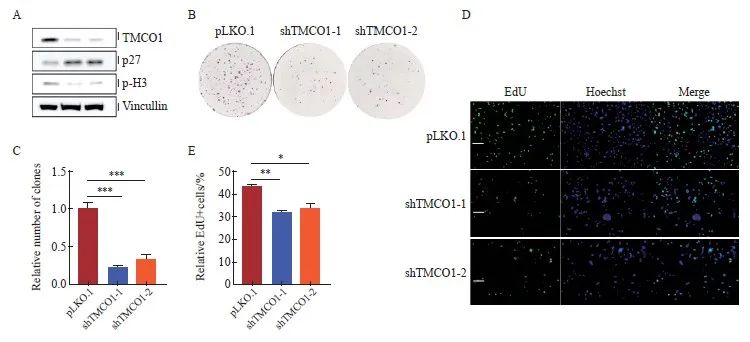
图3 敲减TMCO1抑制宫颈癌HeLa细胞的增殖
Fig. 3 Knocking down TMCO1 inhibited the proliferation of cervical cancer HeLa cells
A: Two independent shRNAs targeting the TMCO1 gene sequence were designed and cloned into the pLKO.1 lentiviral vector, which was used to infect the cervical cancer cell line HeLa. After puromycin selection, cells with stable knockdown of TMCO1 were obtained, and cells infected with the empty vector (pLKO.1) virus were used as controls. The cells were collected and the knockdown of TMCO1, as well as the phosphorylation of cell cycle inhibitory protein p27 and histone H3 were detected by IB. B, C: The above TMCO1 stable knockdown cells and control cells were seeded into 6-well plates at 1 000 cells/well, and the number of clones formed was counted after 10 days of culture. D, E: The above TMCO1 stable knockdown cells and control cells were EdU labeled to detect the number of cells undergoing active DNA synthesis. *: P<0.05, **: P<0.01, ***: P<0.001, compared with empty vector (pLKO.1).
2.3 过表达TMCO1促进HeLa细胞迁移,敲减TMCO1抑制HeLa细胞迁移
将上述获得的稳转Flag-TMCO1细胞及对照细胞(EV),按照实验步骤要求接种于小室(chamber)进行transwell细胞迁移实验。24 h后,甲醛固定结晶紫染色,显微镜下观察、拍照并统计迁移的细胞数量。以感染空载体(EV)的细胞转移数为1.0,获得稳转Flag-TMCO1细胞转移数字的相对值。宫颈癌HeLa细胞过表达TMCO1后,迁移能力显著增加(P<0.001,图4A、B)。
将上述的TMCO1稳定敲减细胞及对照细胞(pLKO.1),按照实验步骤要求接种于小室(chamber)进行transwell细胞迁移实验。24 h后,甲醛固定结晶紫染色,显微镜下观察、拍照并统计迁移的细胞数量。以对照细胞(pLKO.1)的细胞转移数为1.0,获得TMCO1稳定敲减细胞转移数字的相对值。敲减TMCO1显著抑制宫颈癌HeLa细胞的迁移(P<0.001,图4C、D)。
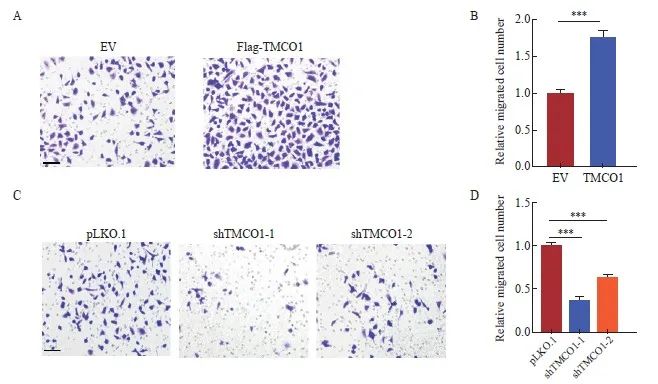
图4 TMCO1促进宫颈癌HeLa细胞的迁移
Fig. 4 TMCO1 promoted the migration of cervical cancer HeLa cells
A, B: The stably transfected TMCO1 cells and control cells (EV) obtained in Figure 2 were inoculated into chambers according to the experimental steps for transwell cell migration assay. After 24 hours, the cells were fixed with formaldehyde and stained with crystal violet, and the number of migrated cells was observed and photographed under a microscope and the number of migrated cells was counted. C, D: The TMCO1 stable knockdown cells and control cells (pLKO.1) in Figure 3 were inoculated into the chamber according to the experimental steps for the transwell cell migration experiment. After 24 hours, the cells were fixed with formaldehyde and stained with crystal violet. The number of migrated cells was observed and photographed under a microscope, and the number of migrated cells was counted. ***: P <0.001, compared with empty vector (EV or pLKO.1).
2.4 TMCO1调节细胞的黏附和相关信号转导通路
收取上述获得的稳转Flag-TMCO1细胞及对照细胞(EV),制备细胞裂解液并进行胰蛋白酶解后,上机进行液相色谱串联质谱分析。收集数据进行主成分分析(principal component analysis),可见2个Flag-TMCO1稳定表达细胞系样本及3个对照细胞(EV)样品,分别聚类在一起,提示TMCO1稳定表达引起蛋白质组学水平的显著变化。对获得的蛋白表达谱数据进行定量分析,对所得到的差异蛋白进行分析并绘制火山图,将有显著性差异的代表性蛋白标注(图5A、B)。
将Flag-TMCO1稳定表达细胞及对照细胞(EV)蛋白表达组差异蛋白进行GSEA-KEGG通路富集分析,显示Flag-TMCO1稳定表达细胞中与细胞外基质-黏附及磷脂酰肌醇3-激酶(phosphoinositide3-kinase,PI3K)/蛋白激酶B (protein kinase B,AKT)信号转导通路激活,而核糖体相关等通路受到抑制(图5C、D)。
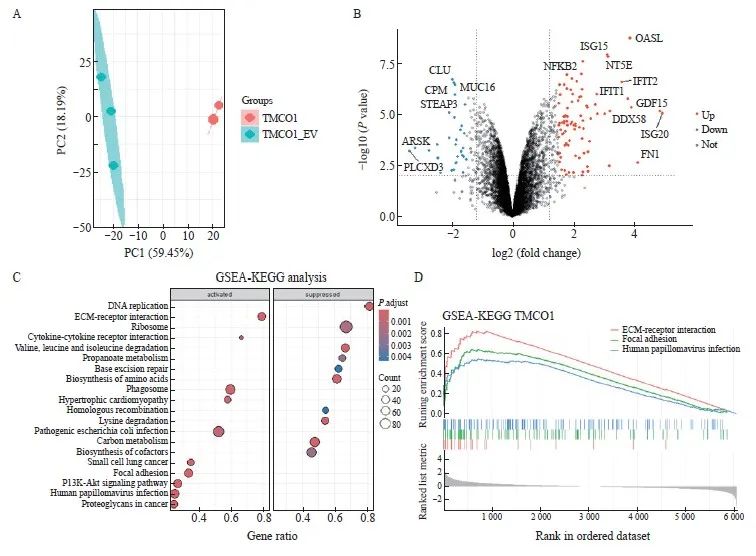
图5 TMCO1调节细胞的黏附和信号转导
Fig. 5 TMCO1 regulated cell adhesion and signaling transduction
A: The Flag-TMCO1 cells and control cells (EV) in Figure 2 were collected, cell lysates were prepared and trypsinized, and then analyzed by liquid chromatography-tandem mass spectrometry. Data were collected for principal component analysis, and it can be seen that the two Flag-TMCO1 stable expression cell line samples and the three control cell (EV) samples were clustered together, indicating that the stable expression of TMCO1 caused significant changes in the proteomic level. B: The differentially expressed proteins obtained by quantitative analysis of the protein expression profile data obtained in (A) were analyzed and a volcano plot was drawn, and representative proteins with significant differences were annotated. C, D: GSEA‑KEGG pathway enrichment analysis was performed on the differentially expressed proteins in the Flag‑TMCO1 stable expression cells and control cells (EV) protein expression groups, showing that pathways related to extracellular matrix-adhesion and PI3K-AKT signaling were significantly upregulated in Flag‑TMCO1 stable expression cells, while ribosome‑related pathways were downregulated.
3 讨 论
宫颈癌发病率和死亡率高,严重威胁女性健康。尽管早期宫颈癌通过手术或放化疗等能达到较好的效果,但是晚期、转移和复发宫颈癌治疗手段有限,患者预后差,肿瘤的侵袭和转移性增强是治疗效果不佳的主要原因。因此,探究宫颈癌侵袭、转移等分子机制,寻找新的治疗靶点,对改善患者的预后具有重要的意义。
研究[11-12]表明,内质网膜功能异常导致的细胞钙稳态失衡与肿瘤的发生、发展密切相关。作为调节细胞钙稳态的重要分子,迄今未见任何研究探讨TMCO1与宫颈癌之间的可能关系。我们的研究表明,TMCO1的表达与宫颈癌患者的预后呈负相关,进一步通过CCK-8实验、克隆形成实验、EdU标记实验提示,TMCO1促进宫颈癌HeLa细胞的增殖,通过transwell实验提示, TMCO1促进宫颈癌HeLa细胞的迁移。宫颈癌HeLa细胞敲减TMCO1后,细胞周期抑制蛋白p27表达增加,组蛋白H3的磷酸化减少。有研究表明p27能够通过结合或抑制细胞周期蛋白或周期蛋白依赖性激酶,阻止细胞从G1期进入S期,与宫颈癌的发展、分化程度及淋巴结转移有关,p27表达阳性的宫颈癌患者其预后优于p27表达阴性的患者。组蛋白H3磷酸化是一种重要的表观遗传修饰方式,可通过改变组蛋白的结构和稳定性,参与细胞分化、增殖、DNA损伤修复等生物学过程,与肿瘤发生、发展相关。
TMCO1在其他肿瘤中的研究也刚起步。Li等[13]研究发现,TMCO1表达在膀胱尿路上皮癌发展过程中逐步下调,TMCO1过表达抑制裸鼠移植瘤的大小,被认为是个抑癌因子,而且是通过抑制AKT信号转导通路实现的,与我们的研究结果正好相反。但Zheng等[14]却研究发现TMCO1在结肠癌组织中表达水平增加,并促进肿瘤的生长和耐药。Gao等[15]也发现TMCO1在胶质瘤中表达上调,并促进肿瘤细胞增殖与诱导上皮间质转化。Yang等[16]同样发现,肺腺癌组织中TMCO1的免疫组织化学染色阳性率明显高于癌旁组织,并且抑制TMCO1表达水平对肺腺癌细胞的转移能力产生负调控。Sun等[17]在今年刚发表的文章中也提出,TMCO1可以通过调控上皮-间质转化(epithelial-mesenchymal transition,EMT)促进卵巢癌的进展,与我们的研究结果一致。
TMCO1如何促进宫颈癌HeLa细胞的增殖和迁移?我们的研究显示Flag-TMCO1稳定表达细胞中与细胞外基质-黏附及PI3K/AKT信号转导通路显著活化。增殖失控是肿瘤细胞的基本特征,其生物学基础是细胞周期调控机制的紊乱,涉及多种信号分子的调节, PI3K/AKT信号转导通路便是其一[18]。PI3K/AKT信号转导通路参与调控肿瘤细胞的增殖凋亡、迁移侵袭、细胞周期、血管生成等多个过程,在肿瘤的发生、发展中发挥了重要作用[19]。EMT是肿瘤细胞侵袭迁移的发生机制之一,与上皮源性肿瘤浸润及转移密切相关,研究发现PI3K/AKT信号转导通路能够调控EMT,从而影响HeLa细胞的迁移侵袭[20]。因此TMCO1高表达可能通过PI3K/AKT信号转导通路调控EMT,进而影响HeLa细胞的增殖、迁移和侵袭。另外,我们的研究还显示Flag-TMCO1稳定表达细胞中与核糖体相关等信号转导通路受到抑制,核糖体是细胞内蛋白质合成重要的细胞器,核糖体生物合成是细胞内重要的生物学过程,已有研究表明核糖体生物合成的异常,包括核糖体数量的增加和修饰的改变可能会影响肿瘤的发生[21]。核糖体生物合成在肿瘤中的异常取决于原癌基因和抑癌基因的改变以及细胞内特定信号转导通路的激活,这些信号转导通路包括RAS/RAF/MEK/ERK、MYC和PI3K/AKT/mTOR。EMT、核糖体生物合成和核糖体RNA转录调控之间的联系,有研究[22-23]显示,在人乳腺癌MCF7细胞中,加入外源性核糖体可诱导EMT,伴以ERα抑制为标志的亚型转分化。因此TMCO1高表达也可能通过核糖体相关通路调控EMT,进而影响HeLa细胞的增殖、迁移和侵 袭。
TMCO1在不同的肿瘤中似乎表现出不同的作用,我们也首次阐述了TMCO1在宫颈癌中的作用,与大多数肿瘤研究结果相似,TMCO1表现为促癌因子的作用,可能成为宫颈癌治疗的靶点,机制探索中除以往文献中有提及的细胞外基质-黏附及PI3K-AKT信号外,我们还发现可能与核糖体相关等通路有联系,具体的促进增殖和迁移的机制还有待于进一步发掘。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
陈珣:完成实验,撰写文章。郑真霞:参与实验。
阮雪茹:审核、修改文章。
[参考文献]
[1]JEMAL A, SIEGEL R, XU J Q, et al. Cancer statistics. Ca cancer J clin[J]. CA A Cancer J Clin, 2010, 60(5): 277-300.
[2]SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2016[J]. CA Cancer J Clin, 2016, 66(1): 7-30.
[3]CAO W, CHEN H D, YU Y W, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J, 2021, 134(7): 783-791.
[4]GROENENDYK J, MICHALAK M. Interplay between calcium and endoplasmic reticulum stress[J]. Cell Calcium, 2023, 113: 102753.
[5]LI W, OUYANG Z, ZHANG Q, et al. SBF-1 exerts strong anticervical cancer effect through inducing endoplasmic reticulum stress-associated cell death via targeting sarco/endoplasmic reticulum Ca(2+)-ATPase 2[J]. Cell Death Dis, 2014, 5(12): e1581.
[6]WANG Q C, ZHENG Q X, TAN H Y, et al. TMCO1 is an ER Ca(2+) load-activated Ca(2+) channel[J]. Cell, 2016, 165(6): 1454-1466.
[7]ZHANG N N, TANG M, WEN M R, et al. Expression, purification and characterization of TMCO1 for structural studies[J]. Protein Expr Purif, 2021, 179: 105803.
[8]SUN Z S, ZHANG H, WANG X, et al. TMCO1 is essential for ovarian follicle development by regulating ER Ca2+ store of granulosa cells[J]. Cell Death Differ, 2018, 25(9): 1686-1701.
[9]LI J W, LIU C Z, LI Y H, et al. TMCO1-mediated Ca2+ leak underlies osteoblast functions via CaMKⅡ signaling[J]. Nat Commun, 2019, 10(1): 1589.
[10]BATCHELOR-REGAN H, XIN B Z, ZHOU A M, et al. From disease description and gene discovery to functional cell pathway: a decade-long journey for TMCO1[J]. Front Genet, 2021, 12: 652400.
[11]ZHENG S L, WANG X W, ZHAO D, et al. Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications[J]. Trends Cell Biol, 2023, 33(4): 312-323.
[12]YANG X, ZHUANG J, SONG W L, et al. Mitochondria-associated endoplasmic reticulum membrane: overview and inextricable link with cancer[J]. J Cell Mol Med, 2023, 27(7): 906-919.
[13]LI C F, WU W R, CHAN T C, et al. Transmembrane and coiled-coil domain 1 impairs the AKT signaling pathway in urinary bladder urothelial carcinoma: a characterization of a tumor suppressor[J]. Clin Cancer Res, 2017, 23(24): 7650-7663.
[14]ZHENG S L, ZHAO D, HOU G X, et al. iASPP suppresses Gp78-mediated TMCO1 degradation to maintain Ca2+ homeostasis and control tumor growth and drug resistance[J]. Proc Natl Acad Sci U S A, 2022, 119(6): e2111380119.
[15]GAO L, YE Z, LIU J H, et al. TMCO1 expression promotes cell proliferation and induces epithelial-mesenchymal transformation in human gliomas[J]. Med Oncol, 2022, 39(5): 90.
[16]YANG C, WANG Y, BAI J Q, et al. Mechanism of transmembrane and coiled-coil domain 1 in the regulation of proliferation and migration of A549 cells[J]. Oncol Lett, 2020, 20(5): 159.
[17]SUN G Y, GONG S, LAN S W, et al. TMCO1 regulates cell proliferation, metastasis and EMT signaling through CALR, promoting ovarian cancer progression and cisplatin resistance[J]. Cell Mol Biol, 2024, 70(1): 99-109.
[18]殷苏威, 张裕民, 郭 丽, 等. Irisin通过PI3K/AKT/Cyclin D1途径促进宫颈癌HeLa细胞增殖的机制研究[J]. 中国计划生育和妇产科, 2022, 14(5): 100-105.
YIN S W, ZHANG Y M, GUO L, et al. The mechanism research of Irisin promoting the proliferation of cervical cancer HeLa cells through PI3K/AKT/Cyclin D1 pathway[J]. Chin J Fam Plan Gynecotokol, 2022, 14(5): 100-105.
[19]CHEN L, LIU D M, YI X F, et al. The novel miR-1269b-regulated protein SVEP1 induces hepatocellular carcinoma proliferation and metastasis likely through the PI3K/Akt pathway[J]. Cell Death Dis, 2020, 11(5): 320.
[20]DAI B L, YU R Z, FAN M Y, et al. HMQ-T-F2 suppresses migration of the human cervical cancer HeLa cells by reversing EMT via the PI3K/Akt signaling pathway[J]. Oncol Rep, 2019, 42(4): 1451-1458.
[21]PELLETIER J, THOMAS G, VOLAREVIĆ S. Ribosome biogenesis in cancer: new players and therapeutic avenues[J]. Nat Rev Cancer, 2018, 18(1): 51-63.
[22]PRAKASH V, CARSON B B, FEENSTRA J M, et al. Ribosome biogenesis during cell cycle arrest fuels EMT in development and disease[J]. Nat Commun, 2019, 10(1): 2110.
[23]KUDO M, ANAM M B, ISTIAQ A, et al. Ribosome incorporation induces EMT-like phenomenon with cell cycle arrest in human breast cancer cell[J]. Cells Tissues Organs, 2022, 211(2): 212-221.


